Retinal
edema threatening or involving the macula is an important visual consequence of
abnormal retinal vascular permeability in diabetic retinopathy1.
Focal/grid photocoagulation has been the mainstay of treatment since its
benefit was demonstrated in the Early Treatment Diabetic Retinopathy Study
(ETDRS) in 19852. However, especially in macular edema laser
treatment is not always beneficial3. Most of the retinal damage that
characterizes the disease is believed to result from breakdown of the inner
blood retinal barrier mediated by numerous growth factors such as vascular
endothelial growth factor (VEGF)4,5. Based on these facts anti-VEGF
agents like Pegaptanib sodium and Ranibzumab have been evaluated for diabetic
macular edema in Phase II randomized trials6,7. One such VEGF
inhibitor is Bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA), a
US Food and Drug Administration approved full-length humanized monoclonal
antibody that until recently was used for the treatment of metastatic
colorectal cancer8. As compared to Pegaptanib, which is a selective
inhibitor of VEGF 165, Bevacizumab inhibits all active isoforms of VEGF.
Intravitreal Bevacizumab is currently being evaluated for use in macular edema
following central retinal vein occlusion (CRVO), wet age-related macular
degeneration (ARMD), rubeosis iridis and proliferative diabetic retinopathy
(PDR)9-12. It seems reasonable to assume that VEGF inhibitors will
also be beneficial in diabetic macular edema. Although intravitreal use of
Bevacizumab is an off-label option, its use has risen exponentially in the
recent past due to its efficacy and economic feasibility. However, there are
very few studies to-date showing the beneficial effect of intravitreal
Bevacizumab for persistent diffuse diabetic macular edema13-15.
The
purpose of this study was to evaluate the beneficial effect of intravitreal
injection of Bevacizumab on visual acuity in diabetic macular edema.
MATERIAL AND METHODS
This was a prospective,
interventional, non-comparitive case series carried out at the Department of
Ophthalmology, Jinnah Hospital Lahore. The study was carried out over a period
of six months from May 2010 to October 2010. Twenty eyes of twenty patients
were included. The sampling technique was non-probability convenience sampling.
We included diabetic patients of all ages and both sexes having
non-proliferative diabetic retinopathy with diffuse, clinically significant
diabetic macular edema, which was previously treated with grid laser (more than
six months ago). However, the following patients were excluded: those having
only focal macular edema attributable to focal leaks from microaneurysm;
patients with any other macular pathology like ARMD or any vascular occlusive
disease affecting the macula; those previously treated with pan retinal
photocoagulation (PRP) and grid laser within last six months; those with
angiographic evidence of widening or irregularity of the foveal avascular zone
suggestive of ischemic maculopathy; and patients with uncontrolled diabetes,
hypertension and/or chronic renal failure.
At each visit, complete eye examination was performed,
including best-corrected visual acuity using Snellen’s testing, slit-lamp
examination, intraocular pressure measurement, stereoscopic biomicroscopy of
the retina using a 78-diopter lens, fluorescien angiography (only on the first
and last visit) and fundus photography of the macular area.
Patients were informed regarding the experimental
nature of the study and written informed consent was obtained from all patients
and official permission was taken from the hospital’s ethics committee.
Injection Technique: All intravitreal injections were
performed under topical anesthesia. Intravitreal Bevacizumab injection was
prepared and dispensed by the pharmacy at Shaukat Khanum Memorial Cancer
Hospital, Lahore at the concentration of 1.25mg/0.05ml. The lid was prepared
with 5% povidone-iodine applied directly to the eye, and Bevacizumab was
injected into the mid-vitreous 3.5mm posterior to the limbus in pseudophakic
eyes and 4.0 mm posterior to the limbus in phakic eyes with a tuberculin
syringe and 27-gauge needle. Topical ciprofloxacin eye drops were applied four
times daily for one week.
Follow-up visits were at 1 week after injection, and
then at 1 month and 3 months.
Only one eye per subject was
treated. All data were analyzed using SPSS 13.0 for windows. The paired t-test
was used for comparison of preoperative and postoperative BCVA. For all
statistical tests a p value of <0.05 was considered statistically
significant.
RESULTS
In this
study, 20 eyes of 20 patients with diabetic macular edema were studied. Of
these 12 (60%) were males and 8 (40%) females. The age range was from 45 to 67
years with a mean of 59.2 ± 6.0 years. (Table 1)
All patients
had diffuse, clinically significant macular edema at baseline for which they
had received grid laser photocoagulation at least 6 months before injection.
All patients completed 12 weeks of follow-up.
The
glycosylated hemoglobin (HbA1c) averaged 6.0 ± 1.3 before starting the study.
Pre-injection,
there was 1 (5%) eye with best-corrected visual acuity(BCVA) better than or
equal to 6/18, 10 eyes (50%) with VA between 6/24 and 6/60 and 9 (45%) with VA
below 6/60.
At the
first post-injection week, no changes were observed in the BCVA.
At
first post-injection month, 2(10%) eyes had BCVA better than or equal to 6/18;
15(75%) between 6/24 and 6/60 and in 3(15%) eyes the vision was worse than
6/60.
Three
months after the injection, again 2 (10%) eyes had BCVA better than or equal to
6/18. However, the number of eyes with BCVA between 6/24 and 6/60 were 12(60%),
while 6(30%) eyes had BCVA worse than 6/60. Thus twelve weeks after the
injection, some regression of the increase in visual acuity was noted (Fig. 1).
Table 1: Clinical characteristics
patients with diabetic macular edema at baseline n = 20
|
Gender |
|
|
Male |
12 |
|
Female |
8 |
|
Age (years), Mean ± SD |
59.2 ± 6 |
|
Glycosylated hemoglobin (HbA1c, %) |
6.2 ± 1.3 |
Table 2: Mean intraocular pressures of patients
before and after intravitreal bevacizumab injection n = 20
|
|
Mean IOP (mmHg) |
|
Pre-injection |
16.2 ± 2.6 |
|
One week |
15.8 ± 2.2 |
|
One month |
16 ± 2.3 |
|
Three months |
16.1 ± 2.2 |
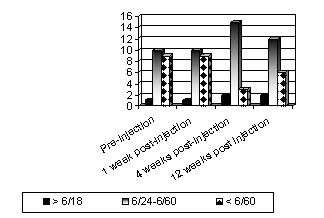
Fig. 1: Graphical representation of pre and post-injection visual
acuities
There
was statistically significant difference in the pre and post injection visual
acuity of the patients. Thus, in comparing the visual acuities at one month and
3 months the p value is less than 0.05.
No
significant increase of IOP was observed throughout the study (Table 2).
Mild anterior chamber cellular
reaction was observed in 3 eyes (10%), however the inflammation resolved within
a week with topical corticosteroid. Other injection related adverse events such
as endophthalmitis, vitreous hemorrhage and retinal detachment were not
observed.
DISCUSSION
Diabetic
retinopathy is the leading cause of blindness in patients aged more than 50
years in our country. 16 The intravitreal injection of Bevacizumab
has been met with great enthusiasm especially for patients with neovascular
age-related macular degeneration. A significant improvement has also been
reported in macular edema secondary to other conditions such as CRVO. It was
also found that the concentration of VEGF increased and correlated with the
severity of macular edema in patients with DME17.
In
light of this information we decided to conduct a prospective, hospital-based
study to investigate the visual outcome after intravitreal Bevacizumab
injection in patients with chronic diffuse diabetic macular edema unresponsive
to previous grid laser photocoagulation. Bevacizumab has attracted interest
because of its low cost; however systemic safety is of concern18,19.
The
results of our study showed that intravitreal Bevacizumab is useful in increasing
visual acuity in patients with diffuse diabetic macular edema. There was a
statistically significant increase in the VA at 4 and 12 weeks after the
injection. Our results are comparable with those of Hartitoglou et al13.
The
observed slight reduction of the increase in visual acuity at the limited
12-week follow-up is also consistent with the findings of Haritoglou. This
decrease also hints that repeated Bevacizumab injections may be necessary.
Many
clinical investigators have found that an intravitreal injection of
Triamcinolone (TA) may reduce macular edema. However, the intravitreal use of
TA may lead to complications such as increased IOP, progression of cataract and
endophthalmitis. 16, 20 However unlike the eyes treated with
triamcinolone, there was no significant rise in the IOP. These results are
comparable to those of Toshihiko and associates14.
A
limitation of the present investigation is the short follow-up, due to which
the long-term safety and efficacy of the treatment could not be assessed. Other
limitations are the lack of a control group, but it can be argued that the
enrolled eyes served as their own controls because the pre and post-injection
visual acuities of patients were compared. Thirdly, the visual acuity was
measured on a Snellen’s chart as opposed to the more standardized and accepted
ETDRS chart. However, all eyes were tested with the same correction throughout
the follow-up period. The strengths are prospective design and careful
follow-up. Most of the studies on the intravitreal injection of Bevacizumab
were designed as retrospective analysis14.
Many
clinical investigators have found that an intravitreal injection of TA may
reduce macular edema. However, the intravitreal use of TA may lead to
complications such as increased IOP, progression of cataract and
endophthalmitis16,20.
Recent studies have shown that
the combination of laser photocoagulation with intravitreal Bevacizumab may
improve BCVA and retinal thickness more than laser photocoagulation alone or
intravitreal Bevacizumab alone for DME21, 22.
CONCLUSION
The positive results of this
prospective, non-randomized study are quite promising and suggest the need for
a longer, prospective randomized studies to evaluate the long-term safety and
efficacy of intravitreal Bevacizumab.
Author’s affiliation
Dr.
Tehmina Jahangir
Senior
Registrar
Eye
Unit I,
IMC/JHL, Lahore
Professor
Samina Jahangir
Professor
and Head
Department
of Ophthalmology
AIMC/JHL, Lahore.
Dr.
Haroon Tayyab
Registrar
Eye Unit 1, AIMC/JHL, Lahore.
Dr.
Uzma Hamza
Assistant
Professor
Eye
Unit I, AIMC/JHL, Lahore
REFERENCE
1.
American Academy of
Ophthalmology. Diabetic Macular edema. In: Basic and Clinical Science Course:
Section. 2009; 12: 113-4.
2.
Early Treatment Diabetic
Retinopathy Study Research Group. Photocoagulation for diabetic macular
edema: Early Treatment Diabetic
Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103: 1796-806.
3.
Diabetic Retinopathy
Clinical Research Network. A randomized trial comparing intravitreal
triamcinolone acetonide and focal/grid photocoagulation for diabetic macular
edema. Ophthalmology. 2008; 115: 1447-59.
4.
Adamis AP, Miller JW,
Bernal MT, et al. Increased vascular
endothelial growth factor levels in the vitreous of eyes with proliferative
diabetic retinopathy. Am J Ophthalmol. 1994; 118: 445-50.
5.
IshidaS, Usui T, Yamashiro K. VEGF
164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;
44: 2155-62.
6.
Cunningham ET, Adamis AP, Altaweel M, et al. A phase II randomized double-masked trial of pegaptanib, an
anti-vascular endothelial growth factor aptamer, for diabetic macular edema.
Ophthalmology. 2005; 112: 1747-57.
7.
Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibzumab
in patients with center-involving clinically significant diabetic macular
edema. Ophthalmology. 2006; 113: 1706-12.
8.
Marshall J. The role of bevacizumab
as first-line therapy for colon cancer. Semin Oncol. 2005; 32: S43-S47.
9.
Spaide RF, Laud K, Fine HF,
et al. Intravitreal bevacizumab
treatment of choroidal neovascularisation secondary to age-related macular
degeneration. Retina. 2006; 26: 383-90.
10.
Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related
macular degeneration. Ophthalmology. 2006; 113: 363-72.
11.
Spaede RF, Fisher YL.
Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic
retinopathy complicated by vitreous hemorrhage. Retina. 2006; 26: 275-8.
12.
Oshima Y, Sakaguchi H, Gomi F, et al. Regression of iris neovascularization after intravitreal
injection of bevacizumab in patients with proliferative diabetic retinopathy.
Am J Ophthalmol. 2006; 142: 155-8.
13.
Haritoglou C, Kook D, NeubauerA, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse
diabetic macular edema. Retina. 2006; 26: 999-1005.
14.
Nagasawa T, Naito T, Matsushita, et al. Efficacy of intravitreal bevacizumab (Avastin) for short-term
treatment of diabetic macular edema. The Journal of Medical Investigation.
2009; 56: 111-5.
15.
Ozkiris A. Intravitreal Bevacizumab
(Avastin) for primary treatment of diabetic macular edema. Eye. 2009; 23: 616-20.
16.
Mehmood K, Malik BA, Khan MT et al. Visual Effects of Intravitreal Triamcinolone Acetonide Injection
in Patients with refractory diabetic macular edema. Pak J Ophthalmol. 2010; 26:
193-6.
17.
Funatsu N, Yamashita H, Sakata K, et al. Vitreous levels of vascular endothelial growth factor and
intracellular adhesion molecule 1 are related to diabetic macular edema.
Ophthalmology. 2005; 112: 806-16.
18.
Rosenfeld PJ. Intravitreal Avastin:
the low cost alternative to Lucentis? Am J Ophthalmol. 2006; 142: 141-3.
19.
Gillies MC: What we don’t know
about Avastin might hurt us. Arch Ophthalmol. 2006; 124: 1478-9.
20.
Bhavsar AR, Glassman AR.
DRCRnet Group, SCORE Study Group: The risk of endophthalmitis following
intravitreal triamcinolone injection in the DRCRnet and SCORE clinical trials.
Am J Ophthalmol. 2007; 144: 454-6.
21.
Maia Jr OO, Takahashi BS, Costa RA, et al. Combined laser and intravitreal triamcinolone for proliferative
diabetic retinopathy and macular edema: one year results of a randomized
clinical trial. Am J Ophthalmol. 2008; 146: 930-41.
22.
Elman
MJ, Aiello LP, Beck RW et al. DRCR network. Randomized trial
evaluating Ranibzumab plus prompt or deferred laser or triamcinolone plus
prompt laser for diabetic macular edema. Ophthalmology. 2010; 117: 1059-60.