Scleritis
is a severe, potentially sight threatening, inflammatory disease involving the
ocular surface. It has been be classified by PG
Watson et al,1 into anterior and posterior. Anterior scleritis can
be diffuse, nodular, necrotizing with inflammation (necrotizing), and
necrotizing without inflammation (scleromalacia perforans). Posterior scleritis2
is characterized by flattening of the posterior aspect of the globe, thickening
of the posterior coats of the eye (choroid and sclera), and retrobulbar edema.
The author states that the anatomical site and clinical appearance of
the disease at presentation reflected its natural history; majority of patients
remain in the same clinical category throughout the course of their disease.
Diffuse anterior scleritis had a lower incidence of visual loss (9%) than
either nodular scleritis (26%)
or necrotizing disease (74%), Patients with necrotizing scleritis were older
than patients in the other groups and more frequently had an associated
systemic disease than patients with either diffuse or nodular disease, 57%.3
According to another study, necrotizing
scleritis is associated with rheumatoid arthritis in most cases and less often
with SLE, Crohn's disease, Behcet's disease and gout.4 Diffuse and
nodular anterior scleritis is less often associated with any systemic disease.
An
underlying infectious etiology has been found to be relatively less common.
According to a study by Gonzalez et al5, out of 500 patients
presenting with scleritis, only 9.4% had an underlying infection with Herpes
virus infection (74%), tuberculosis (10%) and other infections in the remaining
14%.
An autoimmune deregulation in a genetically predisposed host
is presumed to cause scleritis. Inciting factors may include infectious
organisms, endogenous substances, or trauma. The inflammatory process may be
caused by immune complex–related vascular damage (type III hypersensitivity)
and subsequent chronic granulomatous response (type IV hypersensitivity).6
Ocular complications of scleritis, which cause visual loss
and eye destruction, appear as a result of the extending scleral inflammation.
These include peripheral ulcerative keratitis (13 – 14%), uveitis (about 42%),
glaucoma (12 – 13%), cataract (6 – 17%), and fundus abnormalities (about 6.4%).7.They
are most common in necrotizing scleritis, the most destructive type of
scleritis.
Despite
recent advances, its treatment remains a difficult problem. Systemic
immunosuppressive therapy with corticosteroids or immunosuppressive agents or
both is usually required to control the disease. Early therapeutic intervention
is important to prevent ocular complications and to minimize the potential
morbidity and mortality associated with underlying systemic disease. Systemic
corticosteroids in high dosage, either topical orally or in intravenous pulses,
are widely accepted as an effective form of treatment in patients with severe
scleritis.8 But this treatment is often associated with unacceptable
side effects and does not always control scleral inflamma-tion. Side effects
include adrenal suppression, vertigo, psychosis, pseudotumour cerebri, acne,
osteoporosis, myopathy and delayed wound healing.
The
addition of immunosuppressive agents in patients with severe scleritis improves
the ocular outcome and decreases the morbidity associated with systemically
administered corticosteroids.9 Azathio-prine, cyclophosphamide, and
cyclosporine have previously been reported to be effective and safe in the
management of severe ocular inflammation.10
Various studies have reported
the successful use of topical cyclosporine in the treatment of patients with a
variety of ocular inflammatory syndromes resistant to other immunosuppressive
regimens.11,12 Evidence obtained
from these studies supports the efficacy of topical cyclosporine treatment
through its immunomodulatory action, reversing inflammation of the ocular
surface and lacrimal glands. In the present study we report the
therapeutic effect of topical cyclosporine in the treatment of mild to moderate
scleritis.
MATERIAL AND METHODS
This is
a prospective case series of eleven consecutive patients with scleritis who
attended the out patients department of Mughal eye hospital, Lahore, from
January to December 2012. All cases were assessed by the same ophthalmologist.
A detailed history was taken and physical examination performed; distinction
was made between episcleritis and scleritis by the presence of eye pain, local
tenderness over the area of nodule in 6 cases and diffuse purplish hue in 5
cases of scleritis; the conjunctiva could be freely moved over that area and no
blanching of the lesion was achieved by a drop of 10% phenylephrine eye drops.
The cases presenting with episcleritis were excluded from the study. All
patients were graded to have an active anterior non-necrotizing scleritis of
mild to moderate severity without associated anterior or posterior uveitis.
Appropriate investigations including CBC, ESR, Rh – factor, serum ANCA, ACE,
chest X-ray and Mantoux test to exclude the presence of an associated
auto-immune or infectious disease; HLA auto-antibodies and C Reactive Protein
testing was not done due to economic constraints.
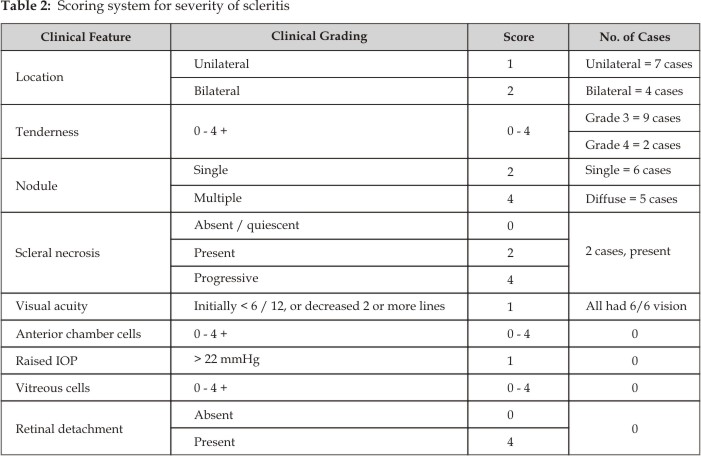
The
clinical features of the patients are summarized in (Table 1). There were seven
females between ages of 18 – 65 years and four males with the age of 45 years.
A subjective grading system, analogous to that previously described for
patients with uveitis and retinal vasculitis was used (Table 2).13 A
scleritis score was calculated when the patients first presented, then at each
follow-up visit and at the end of the study (Table 3). Improvement was defined
as a decrease in the total score of greater than 2 and resolution at a total
score of less than or equal to 4.
All patients were fully
informed regarding the proper use and expected side-effects of topical
cyclosporine which was then started as 1% twice /day (freshly prepared from
cyclosporin capsules, 50 mg BIORAL; the water-miscible gel from 2 capsules
mixed with 5cc distilled water) along with preservative – free artificial tears
4 x / day (Biolan gel from Stullin Pharma, Germany). Treatment was started and
patients were reviewed weekly to note their subjective and objective
improvement. The therapy was conti-nued for 2 months in mild cases (Scleritis
score 6 – 7) and 3 months in moderate cases (Scleritis score = > 8). Then it
was gradually tapered by reducing the strength of Cyclosporin drops to 0.075%,
twice daily for 1 week, then once daily for one week, then on alternate day for
a week and then it was finally stopped.
RESULTS
Associated
active rheumatoid arthritis was found in only 2 female cases who were 60 years
old. No other systemic auto-immune or infectious disease was present in any
other case.
In all
cases, the subjective improvement of eye pain was noted in the first week of
therapy and objectively, reduction in scleral injection and tenderness improved
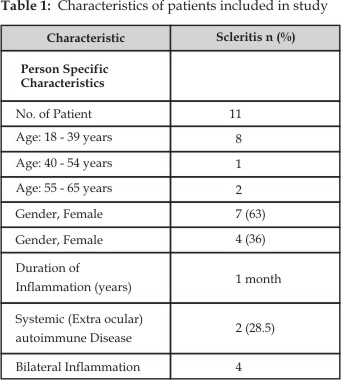
within 2 weeks of therapy. The scleritis scoring system is detailed in Table 2,
and Table 3 summarizes the scleritis score for each patient at presentation and
at four weeks after the commencement of treatment. In all patients
there was a significant decrease in the scleritis score at four weeks. This
decrease in the scleritis score was maintained over the subsequent observation
period. None of the cases developed scleral thinning, uveitis or a raised IOP
during or after the termination of therapy. The mean duration of treatment was
2 months in mild cases and 3 months in moderately severe cases. No recurrence
of scleritis was noted in 9 cases after stopping the therapy. Two cases (18%)
stopped therapy abruptly after one month only; in
them,
scleritis re-appeared which was again successfully controlled by starting the
same therapy and educating them regarding their proper use and weaning. No
recurrence of symptoms was noted in any case over one year follow-up after
stopping the therapy.
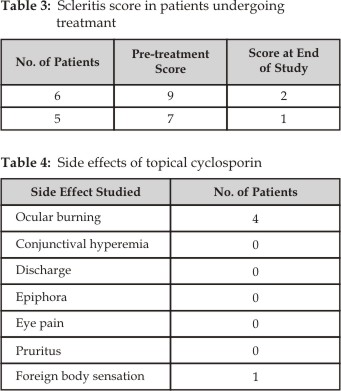
Cyclosporin eye drops were well
tolerated by all patients. Table 4 summarizes the side effects looked for in
the study. The only problem noted was stinging and a burning sensation on
instillation of drops which subsided after instilling preservative-free tear
drops after 10 minutes of instillation of cyclosporin eye drops. No other side
effect was noted.
DISCUSSION
All
eleven patients included in the study responded to the use of cyclosporine
(freshly prepared at our hospital pharmacy). A subjective improvement of eye pain
was noted within one week following the start of therapy and improvement of
scleral injection and tenderness was noted after two weeks of therapy.
It has
been increasingly recognized as an effective therapeutic agent in the
management of a variety of autoimmune diseases. It has been effectively used
topically in ocular surface disorders like vernal keratoconjunctivitis, dry eye
syndrome. Inflammatory eye disease, particularly uveitis, is often well controlled
by the regular use of systemic cyclosporin14.
Cyclosporin
represents the prototype of a new class of drug that appears to work, at least
in part, by acting at the level of cytokine production by immune cells.15 The
selective ability of cyclosporine to interfere with the action of interleukin-2
makes it an appropriate agent for the treatment of diseases mediated by T
cells. Although the immune-pathogenesis of scleritis is not fully understood,
it is believed to be due to immune complex mediated vascular damage to scleral
vessels, with the subsequent generation of a granulomatous reaction. T cells
have an essential role in the formation of such granulomas, and cyclosporine
may act in part by decreasing this component of the inflammatory response.
Multiple
studies on the efficacy of topical cyclosporine for treating inflammatory
ocular surface disorders have consistently shown a beneficial effect of the
drug.14,17 The immune-pathogenic mechanism is complex and involves
an IgE mediated immediate hypersensitivity response as well as a T cell
mediated immune reaction. Animal studies have shown that Cyclosporin has no
intraocular penetration; it concentrates on the ocular surface which enhances
its anti-inflammatory effect after long term use. Even after a year of regular
topical therapy, none to minimal blood concentration and in aqueous taps was
found in rabbits.
A large
study16 of 392 patients with non-infectious anterior scleritis
highlighted various therapeutic options available included NSAIDs, particularly
cox-2 inhibitors (but they result in cardiovascular side effects) in 144
(36.7%), oral or topical steroids in 29 (7.4%), immune modulatory drugs
(systemic cyclophosphamide, azathioprine, methotrexate) in 149 (38.0%),
biologic response modifiers (BRM) in 56 (14.3%), and none (N = 14). Patients
with idiopathic diffuse or nodular scleritis with a low degree of scleral
inflammation or without ocular complications may respond to NSAIDs. Patients
with idiopathic diffuse or nodular scleritis with a high degree of scleral
inflammation may respond to steroids. Patients with diffuse or nodular
scleritis with associated systemic disease may respond to IMT or BRMs. Patients
with necrotizing scleritis may respond to IMT, mainly alkylating agents.
All these systemic therapies
are associated with many side effects. Similarly, topical steroids potentiate
corneo-scleral thinning, raised intra-ocular pressure and cataract. In
comparison, topical cyclosporin has minimal side-effects. Only precaution to be
used is that the beneficial affect is achieved after two weeks of therapy but
the immune process is still active and the therapy has to be continued for at
least two months and then gradually tapered, otherwise the disease flares up if
treatment is stopped abruptly and too early as seen in our two cases.
CONCLUSION
This study
highlights the fact that topical cyclosporine is a potentially useful drug in
the treatment of mild to moderate anterior scleritis; subjective and objective
clinical improvement is achieved within 2-3 weeks of regular usage. Hence, it
is an effective steroid- sparing agent. Unfortunately, its use is complicated
by frequent, mild side effects, like burning and stinging for a short while
after instillation of eye drops and may be associated with recurrence of
disease on suddenly stopping the therapy.14 Patients need to be
informed and educated regarding its appropriate use.
To avoid recurrence of the
disease, therapy has to be continued for at least 2 – 3 months and then
gradually tapered. Another problem with cyclosporine eye drops is that they
have to be made fresh, without preservatives and have a shelf life of one week
only. Despite these limitations we consider that topical cyclosporine is a
useful drug in the management of mild to moderate scleritis, and has a high
therapeutic value in the treatment of this disease.
Author’s
Affiliation
Dr. Sameera Irfan
Mughal Eye Hospital (Trust)
Lahore
Dr. Harris Iqbal
Mughal Eye Hospital (Trust)
Lahore
REFERENCES
1.
Tuft SJ, Watson PG. Progression
of scleral disease. Ophthalmology. 1991; 98: 467-71.
2.
Machado Dde O, Curi AL, Fernandes RS, Bessa TF, Campos WR, Oréfice
F. Scleritis: clinical
characteristics, systemic associations, treatment and outcome in 100 patients.
Arq Bras Oftalmol. 2009; 72: 231-5.
3.
Riono
WP, Hidayat
AA, Rao
NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology.
1999; 106: 1328-33.
4.
Sousa JM, Trevisani VF, Modolo RP, Gabriel LA, Vieira LA, Freitas DD.
Comparative study of ophthalmological and serological manifestations and
the therapeutic response of patients with isolated scleritis and scleritis
associated with systemic diseases. Arq Bras
Oftalmol. 2011; 74: 405-9.
5.
Sainz de la Maza M, Jabbur NS; Foster CS. Severity of scleritis and episcleritis. Ophthalmology. 1994; 101: 389-96.
6.
Sainz de la Maza M, Foster CS, Jabbur NS. Scleritis associated with systemic vasculitic
diseases. Ophthalmology 1995; 102: 687-92.
7.
Durrani K, Zakka FR, Ahmed M, Memon M, Siddique SS, Foster CS. Systemic therapy
with conventional and novel immunomodulatory agents for ocular inflammatory
disease. Ophthalmol. 2011; 56: 474-510.
8.
Raizman M. Corticosteroid therapy of eye
disease. Fifty years later. Arch Ophthalmol.
1996; 114: 1000-1.
9.
Sainz de la Maza M,
Molina N,
Gonzalez-Gonzalez
LA, Doctor PP,
Tauber J,
Foster CS. Scleritis therapy. Ophthalmology.
2012; 119: 51-8.
10.
Carrasco
MA, Cohen
EJ, Rapuano
CJ, Laibson
PR. Therapeutic decision in anterior scleritis:
our experience at a tertiary care eye center. J Fr Ophtalmol. 2005; 28: 1065-9.
11.
Machado
Dde O, Curi
AL, Fernandes
RS, Bessa
TF, Campos
WR, Oréfice
F. Scleritis: clinical characteristics, systemic associations,
treatment and outcome in 100 patients. Arq Bras Oftalmol.
2009; 72: 231-5.
12.
Gumus K, Mirza GE, Cavanagh HD, Karakucuk S. Topical cyclosporine A as a steroid-sparing
agent in steroid-dependent idiopathic ocular myositis with scleritis: a case report and review of
the literature. Eye Contact Lens. 2009; 35: 275-8.
13.
Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing
necrotizing scleritis or peripheral ulcerative keratitis. Ophthalmology. 1984;
91: 1253-63.
14.
Utine CA, Stern M, Akpek EK. Topical Ophthalmic Use of Cyclosporine A. Immunology and Inflammation. 2010; 18: 352-61.
15.
McCluskey PJ, Wakefield D.
Current concepts in the management of scleritis. Aust NZ J Ophthalmol. 1988;
16: 169-76.
16.
Hillenkamp J, Kersten A, Althaus C, Sundmacher R. Cyclosporin A therapy in severe anterior scleritis. 5 severe courses without verification of associated
systemic disease treated with cyclosporine
A. Ophthalmology. 2000; 97: 863-9.
17.
Kaçmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S,
Nussenblatt RB, Rosenbaum JT, Suhler EB, Thorne JE, Jabs DA, Clarke GA, Foster
CS. Cyclosporine
for
ocular inflammatory diseases. Ophthalmology.
2010; 117: 576-84.