Original Article
Association between Hyperhomocysteinemia and
Diabetic Retinopathy
Imran
Ghayoor, Shabana Siddiqui, Ghazala Tabssum
Pak J Ophthalmol 2013, Vol. 29 No.
4
. . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . .. . .. . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . .
|
See
end of article for authors
affiliations …..……………………….. Correspondence
to: Imran
Ghayoor Liaquat
National Hospital Karachi-74800 …..……………………….. |
Purpose: To study the association between
hyperhomocysteinemia (Hcy) and retinopathy among diabetics and non diabetics. Material and Methods: This
Case control study was carried out at the department of Ophthalmology Liaquat
National Hospital Karachi from March 2008 to November 2008. A total of 154
subjects were selected from Eye OPD, out of them 77 were diabetics with early
retinopathy (cases) and 77 were non diabetics and had no history of ocular
diseases (controls). Patients with advance proliferative DR were excluded.
Sample size was calculated with the help of openepi software. Non probability
purposive sampling was done. Results: Serum
Hcy levels measured higher than 12 µmol/L in 69 (85.2%) patients and lower
than 12 µmol/L in 8 (10.9%) patients with diabetes. While serum Hcy levels
were lower than 12 µmol/L in 65 (89.1%) patients and higher than 12 µmol/L in
12 (14.8%) patients of control groups. Serum Hcy levels were significantly
higher in DR patients than non diabetics. According to the findings, serum
Hcy levels more than 12 µmol/L were 47 times more frequent in diabetic
patients with retinopathy than in non diabetics, with odds ratio of 46.71
(95% CI:17.95 to 121.6). Conclusion: A significant association was observed
between hyperhomocysteinemia and DR, with chi square value of 46.79 and P
value 0.0005 at the end of the study. |
Diabetes
mellitus refers to the group of diseases that leads to high blood glucose
levels due to defects in either insulin secretion or insulin action in the body1.
Pakistan has a population of 154 million and more than 10% of its adult
population has diabetes2. According to World Health Organization
(WHO) estimates, there are 177 million diabetics in the world3.
Diabetes
mellitus is characterized by recurrent or persistent hyperglycemia as fasting
plasma glucose level at or above 126 mg/dl, and plasma glucose at or above 200
mg/dl, two hours after a 75 gm oral glucose load as in a glucose tolerance test4.
The current recommended goal for HbA1C in patients with diabetes is < 7.0 %,
which is considered good glycemic control. People with diabetes who have HbA1c
levels within this range have a significantly lower incidence of complications
from diabetes including retinopathy and diabetic nephropathy5,6.
Individuals with diabetes are 25 times more likely to become blind than
individuals without this disease. In many developed countries diabetic
retinopathy (DR) is a leading cause of new cases of visual impairment and
blindness among adults aged 20 – 74 years.8 Among people with type 1
diabetes, about 25% develop DR during the first five year and about 100% within
two decades8. Among people who have type 2 diabetes, about 31% have
retinopathy at diagnosis,8 and more then 60% develop DR during the
first two decades of the disease9.
DR
seems to be essentially a retinal vascular disorder probably beginning in the
capillary bed. Epidemiological studies have shown that the risk and severity of
DR are strongly related to the duration of diabetic mellitus, hyperglycemia and
hypertension, and also but less consistently to hypercholesterolemia and
smoking10. Another study showed an association between
the presence of DR and C677T Polymorphism of the methylenetetrahydrofolate
reductase (MTHFR) gene among patients with type 2 DM11. DR
involves both morphologic and functional changes of retinal capillaries12,13.
Homocysteine (Hcy) is a sulfhydryl amino acid that is considered to play
an important role in vascular injury resulting in the development of peripheral
and coronary arterial disease14.
Hyperhomocysteinemia
may induce endothelial dysfunction and injury followed by platelet activation
and thrombus formation, possibly by increasing oxidative stress;15
therefore, it is conceivable that hyperhomocysteinemia is causally related to
retinal vasculopathy through changes of the retinal vasculature and formation
of microthrombi16. Hyperhomocysteinemia is a strong risk factor for
overall mortality in diabetic patients than among diabetics and non-diabetics17.
So,
plasma Hcy should be assessed in all diabetic patients and any existing
hyperhomocysteinemia should be treated with the aim of reducing the toxic
effect of Hcy and preventing further capillary closure and hypoxia.
This research was an attempt to
study the associ-ation between hyperhomocysteinemia and retinopathy in our
population of diabetics and non diabetics, which may help in early diagnosis,
treatment and prevention of new cases of visual impairment.
MATERIAL AND METHODS
This
case control study was carried out at the department of Ophthalmology, Liaquat
National Hospital Karachi from March 2008 to Nov 2008. A total of 154 subjects
were selected. Sample size was calculated with the help of openepi software.
Non probability purposive sampling was done.
Inclusion
Criteria for case: patients between 20-60 years of either gender, suffering
from DR of duration between 5-15 years, which was diagnosed on fundus
examination using slit lamp. The fasting blood glucose should be >126 mg/dl
or random blood glucose of >200 mg/dl or HbA1c should be between 6.0-9.0
mg%.
Inclusion
Criteria for Controls: patients between 20-60 years of either gender who were
non diabetic and had no history of ocular diseases.
Exclusion
Criteria for cases: Diabetic patients without retinopathy and diabetic patient
with retinopathy but duration of < 5 year. Diabeties with advance diabetic
retonopathy with serum creatinine of >1.5 mg/dl.
Exclusion
Criteria for controls: Patients who refused to get serum homocysteine levels
checked or who did not have serum creatinine level of >1.5 mg/dl as
increased serum creatinine level means there is spurious increase level of
serum homocysteine, so it would not represent the true status of Hcy level.
Patients
who fulfill exclusion and inclusion criteria were collected through
ophthalmology department of Liaquat National Hospital. Controls were matched on
age and gender, were selected from the same OPD, and were not suffering from
diabetes as confirmed by investigations. From all patients serum Hcy levels was
analyzed for determination of association in both groups which were matched
according to the gender and age. Informed consent was taken from all patients
and as there was no active intervention involved, ethical committee approval was
not sought, the hospital approved to bear the cost of tests done for this
study. History, ocular examination (via slit lamp biomicro-scopy through 90D)
and Hcy levels were recorded in a performa. Patients with renal dysfunction
associated with high Hcy levels were excluded from the study.
SPSS-10 was used to analyze
data. Frequency and percentage were computed for categorical values like
gender, DR and Hcy level (>12.0 µmol/L) {5.0 -12.0 µmol/L}. Mean and
standard deviation were computed for quantitative variables like age and
duration of diabetes. Odds ratio was computed to determine the relationship
between DR and hyperho-mocysteinemia using 2x2 table and significance was
evaluated through the confidence interval (CI). P value <0.05 was considered
as significant.
RESULTS
A total
of 154 patients were included in this study, in which 77 patients with DR were
taken as cases and 77 non diabetics with no history of the ocular disease were
taken as control in the study. Controls were matched by age and gender and were
selected from the same OPD.
The
average age of the patients was 42.21±11.95 years (95% CI: 40.31 to 44.11).
Similarly average Hcy level was 16.35 ± 9.83 µmol/l (95%CI: 14.79 to 17.92) and
average duration of diabetes was 8.99 ± 3.44 years (95% CI: 8.21 to 9.77) as
shown in table 1. Age and gender were similar in both groups because of
matching.
Of the
77 diabetes patients, 34 (44.2%) patients were observed with duration of
diabetes 8 to 10 years, 28 (36.7%) patients were with the duration of 5 to 7 years
and 15 (19.5%) were observed with the duration of diabetes 11 to 15 years.

Out of
154 patients, 80 (51.9%) were male and 74 (48.1%) were female with 1.08:1
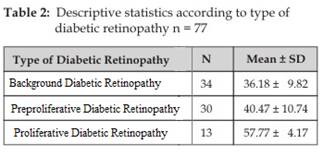
male to female ratio. There were 34 (44.2%)
patients with background diabetic retinopathy (mean age 36.18 ±
9.82 years) and 13 (16.9%) patients with
proliferative diabetic retinopathy (mean age 57.77 ± 4.17 years) and 30 (39%) patients with PPDR (mean age 40.47 ±
10.74 years) as shown in (Table 2).
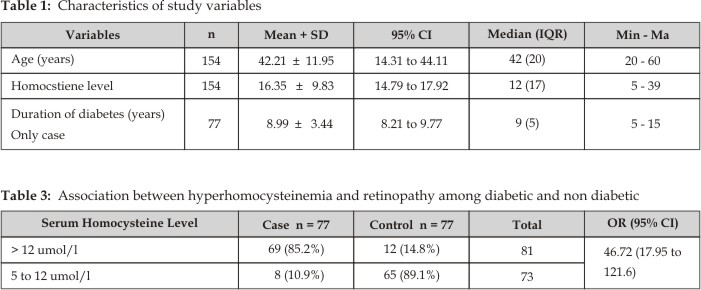
Associations between
hyperhomocysteinemia and DR in diabetics and non diabetics are presented in
table 3. Serum Hcy levels measured higher than 12 µmol/L in 69 (85.2%) patients
and lower than 12 µmol/L in 8 (10.9%) patients of cases. While serum
homocysteine level lower than 12 µmol/L in 65 (89.1%) patients and higher than
12 µmol/L in 12 (14.8%) patients of control groups as presented in Table 3.
Serum homocysteine level was significantly higher in diabetic retinopathy
patients than no diabetics. According to the findings, serum homocysteine level
more than 12 µmol/L. was 47 times more frequent in diabetic patients with
retinopathy than non diabetics (Odds Ratio = 46.71, 95% CI: 17.95 to 121.6).
DISCUSSION
DR is a
leading cause of blindness among patients with DM18. It involves
both morphologic and functional changes of retinal capillaries19,20.
PDR is augmented by retinal hypoxia21. Hyperhomocys-teinemia may
induce endothelial dysfunction and injury following platelet activation and
thrombus formation, possibly by increasing oxidative stress15. So it
is thought that hyperhomocysteinemia is casually related to retinal
vasculopathy through changes of the retinal vasculature and formation of
microthrombi15,17. Oxidative stress is thought to be increased in DM22;
this may make them more susceptible to hyperhomo-cysteinemia induced oxidative
damage.
Hoogeveen
et al looked for an association between the Hcy level and retinopathy among
subjects diabetics and non diabetics. There were 625 numbers of patients. They
defined hyperhomocysteinemia as serum total Hcy level greater than 16 µmol/L.
In their study the prevalence of retinopathy was 9.8% (28/285) in subjects with
normal glucose tolerance, 11.8% (20/169) in those with impaired glucose
tolerance, 9.4% (10/106) in those with newly diagnosed DM, and 32.3% (21/65) in
those with known DM. It was 12.0% (64/534) in subjects with a serum total Hcy
level of 16 µmol/L or less and 16.5% (15/91) in those with a serum total Hcy
level of more than 16 µmol/L. After stratification for DM and adjustment for
age, sex, glycosylated hemoglobin, and hypertension, the odds ratio (95%
confidence interval) for the relation between retinopathy and
hyperhomocysteinemia was 0.97 (95% confidence interval, 0.42 - 2.82) in
non-diabetic patients and 3.44 (95% confidence interval, 1.13 – 10.42) in
diabetic patients with DM, P value was 0.0823.
Ambrosch
et al examined 65 patients with
diabetes; 43 were found to have diabetic neuropathy and this subgroup had
elevated levels of Hcy and a higher prevalence of hyperhomocysteinaemia24.
Vaccaro
et al studied 66
patients with diabetes and found patients with PDR; Hcy was significantly
higher when compared to patients without retinopathy due to the genetic
homozygote C677T mutation which was at least twice as frequent in the diabetic
patients25.
M
Goldstein et al, evaluate the prevalence of hyperhomocysteinemia in diabetic
patients with no DR with non proliferative diabetic retinopathy (NPDR) and with
proliferative diabetic retinopathy (PDR) that study included 179 diabetic
patients and 156 age matched controls with no diabetes and no history of the
ocular disease, who were undergoing routine physical checkups. They were using
high performance liquid chromatography (HPLC) technique for plasma Hcy level
measurement. Hyperhomocysteinemia was defined when Hcy level were higher than
15 µmol/L. The mean plasma homocysteine level was 11.75 ± 0.24 in the control
group, 13.46 ± 0.74 in the no DR group, 14.56 ± 0.64 in the N PDR group and
15.86 ± 1.34 in the PDR group. Mean Hcy levels were significantly elevated in
the NPDR and PDR groups compared to the control group (P=0.001 and <0.0001,
respectively). The prevalence of hyperhomocysteinemia was also higher in the
NPDR and PDR groups compared to the control group (P=0.032 and 0.011, respectively).
No statistically significant difference was found between the no DR and the
control group26.
A total
of 154 patients were included in this study, 77 diabetic patients with DR
including background, non proliferative and proliferative diabetic retinopathy
and 77 age and gender matched controls with no diabetes and non history of
ocular disease were selected from the same OPD. Plasma Hcy levels of all study
participants were measured using Fluorescence
Polarization Immunoassay Technique (FPIT)27.
Serum
homocysteine level measured higher than 12 µmol/L in 69 (85.2%) patients and
lower than 12 µmol/L in 8 (10.9%) patients of cases. While serum homocysteine
level lower than 12 µmol/L in 65 (89.1%) patients and higher than 12 µmol/L in
12 (14.8%) patients of control groups as presented in Table 3. Serum
homocysteine levels were significantly higher in DR patients than non
diabetics. According to the findings, serum homocysteine level more than 12
µmol/L was 47 times more frequent in diabetic patients with retinopathy than
non diabetics, an odds ratio of 46.71 with 95% CI: 17.95 to 121.6. It was
concluded that significant association was observed between
hyperhomocysteinemia and DR, chi square 46.79 and P value 0.0005 at the end of
the study.
It is
considered that a higher plasma level of Hcy in diabetic patients may play a
role in accelerating the micro vascular retinal changes, and may therefore
contribute to the severity of DR.
The prevalence of
hyperhomocysteinemia and mean plasma homocysteine level in DR patients were
higher than in the control group, those patients who have PPDR and PDR have
higher Hcy level than BDR. Therefore, a longer follow up period is needed to
evaluate the long term effects of Hcy levels on the progression of DR.
Hyperhomocysteinemia is one of the contributing factor to micro vascular
angiopathy via thrombus formation in the capillaries and further impairment in
blood supply to the affected tissue. It is necessary that plasma homocysteine
should be assessed in all diabetic patients and that any existing
hyperhomocysteinemia should be treated with the aim of reducing the toxic
effect of Hcy and preventing further capillary closure and hypoxia.
CONCLUSION
Hyperhomocysteinemia may be a
risk factor for retinopathy in patients of diabetes, but probably not in
patients without diabetes and it partially explains the increased risk of micro
vascular angiopathy in diabetic patients and can be used as a marker for the
development of DR.
Author’s Affiliation
Dr.
Imran Ghayoor
Liaquat
National Hospital
Karachi
Dr.
Shabana Siddiqui
Liaquat National Hospital
Stadium Road, Postal Code74800
Karachi
Dr.
Ghazala Tabssum
Liaquat National Hospital
Karachi
REFERENCES
1.
Rother KI. Diabetes treatment bridging the
divide. N Engl J Med. 2007; 356:
1499-501.
2.
Shera AS, Rafique G, Khwaja IA, Baqai S, Khan IA, King H. Pakistan National diabetes survey prevalence of glucose
intolerance and associated factors in North West at Frontier Province (NWFP) of
Pakistan. J Pak Med Assoc. 1999; 49: 206-11.
3.
West S, Sommer A. Prevention of blindness and priorities for the future. Bull
World Health Organ. 2001; 79: 244-8.
4.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes
mellitus and its complications. Part 1: diagnosis and classification of
diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15: 539-53.
5.
Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central
obesity and its atherogenic consequences. The adipose tissue overflow
hypothesis. Int J
Epidemiol. 2007; 36: 220-5.
6.
Genuth S. Insights from the diabetes control and complications
trial / epidemiology of diabetes interventions and complications
study on the use of intensive glycemic treatment to reduce the risk of
complications of type 1 diabetes Endocr Pract. 2006; 12: 34-41.
7.
Jamal-u-Din, Qureshi MB, Khan AJ, Khan MD, Ahmad K. Prevalence of diabetic retinopathy among individuals
screened positive for diabetes in five community-based eye camps in northern
Karachi, Pakistan. J Ayub Med Coll Abbottabad. 2006; 18: 40-3.
8.
American Diabetes Association. Standards of
medical care for patients with diabetes mellitus. Diabetes Care.
2003; 26: 33-50.
9.
Fong DS, Aiello LP, Ferris FL 3rd, Klein R. Diabetic retinopathy. Diabetes Care.
2004; 27: 2540-53.
10.
Tight blood pressure control and risk of macrovascular and
microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective
Diabetes Study Group. BMJ. 1998; 317:
703-13.
11.
Neugebauer S, Baba T, Kurokawa K, Watanabe T. Defective homocysteine metabolism as a risk factor for
diabetic retinopathy. Lancet. 1997; 349: 473-4.
12.
Mandarino LJ. Current hypotheses for the biochemical basis of
diabetic retinopathy Diabetes Care.
1992; 15: 1892-901.
13.
Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Lösche C. Vitreous levels of the insulin-like growth factors I and
II, and the insulin-like growth factor binding proteins 2 and 3, increase in
neovascular eye disease. Studies in non diabetic and diabetic subjects. J Clin
Invest. 1993; 92: 2620-5.
14.
Elias AN, Eng S. Homocysteine concentrations in patients with diabetes
mellitus relationship to micro vascular and macro vascular disease. Diabetes
Obes Metab. 2005; 7: 117-21.
15.
Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J
Med. 1998; 338: 1042-50.
16.
Giugliano D, Ceriello A, Paolisso
G. Oxidative stress
and diabetic vascular complications. Diabetes Care. 1996; 19:
257-67.
17.
Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ, et al. Hyperhomocysteinemia increases
risk of death, especially in type 2 diabetes : 5 year follow-up of the Hoorn
Study. Circulation.
2000; 101: 1506-11.
18.
Clark CM Jr, Lee DA. Prevention and treatment of the complications of
diabetes mellitus. N Engl
J Med. 1995; 332: 1210-7.
19.
Mandarino LJ. Current hypotheses for the biochemical basis of
diabetic retinopathy Diabetes Care.
1992; 15: 1892-901.
20.
Meyer-Schwickerath R, Pfeiffer A, Blum WF, Freyberger H, Klein M, Lösche C. Vitreous levels of the insulin-like growth factors I
and II, and the insulin-like growth factor binding proteins 2 and 3, increase
in neovascular eye disease. Studies in non diabetic and diabetic subjects. J Clin
Invest. 1993; 92: 2620-5.
21.
Pe'er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial
growth factor by retinal cells is a common factor in neovascularizing ocular
diseases. Lab Invest. 1995; 72: 638-45.
22.
Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care.
1996; 19: 257-67.
23.
Hoogeveen EK, Kostense PJ, Jakobs C, Dekker JM, Nijpels G, Heine RJ. Hyperhomocysteinemia increases risk of death,
especially in type 2 diabetes: 5-year follow-up of the Hoorn study. Circulation. 2000; 101: 1506-11.
24.
Ambrosch A, Dierkes J, Lobmann R, Kühne W, König W, Luley C. Relation between homocysteinaemia and diabetic
neuropathy in patients with type 2 diabetes mellitus. Diabet Med. 2001; 18: 185-92.
25.
Vaccaro O, Perna AF, Mancini FP, Iovine C, Cuomo V, Sacco M. Plasma homocysteine and microvascular complications in
type 1 diabetes. Nutr Metab Cardiovasc Dis. 2000; 10: 297-304.
26.
Goldstein M, Leibovitch I, Yeffimov I, Gavendo S, Sela BA, Loewenstein A. Hyperhomocysteinemia in patients
with diabetes mellitus with and without diabetic retinopathy. Eye (Lond).
2004; 18: 460-5.
27.
Leino A. Fully automated measurement of
total homocysteine in plasma and serum on the abbott imx analyzer. Clin Chem. 1999; 45: 569-71.