Mitomycin C (MMC) has been used for treating various ocular
disorders ranging from pterygium to glaucoma. Chen et al1 were the
first researchers to use MMC intraoperatively for refractory glaucoma. Since
then it has become the drug of choice to augment trabeculectomy for effectively
controlling intraocular pressure (IOP) in different types of glaucoma. The
success of MMC has been attributed primarily to its antimetabolitic and antifibrotic
effect shown in numerous clinical2,3 and laboratory studies4,5. The most important postoperative
complications of this procedure are early and late hypotony6-8. In the immediate postoperative state,
increased flow of aqueous through the filtering site has been cited as the
major contributing factor resulting in decreased IOP9. Conversely, this does not explain the late
onset of hypotony (< 6 mm Hg) in some patients undergoing trabeculectomy
with MMC. There is growing evidence from experimental studies that MMC may be
toxic to the ciliary body epithelium, resulting not only in decreased IOP, but
also affecting aqueous humor dynamics and causing a number of other
complications10.
Xia et al observed
swelling of the intracellular mitochondria along with the non-pigmented
epithelium of the ciliary body in rabbit eyes exposed to MMC, signifying its
toxic effect, with decreased aqueous production resulting in hypotony11. In a study by Levy and coworkers,
microscopic examination of rat eyes treated with MMC showed pyknotic nuclei in
conjunction with irregular flattened cells in the ciliary body12. The severity of changes correlated with the
concentration and duration of exposure to MMC. The authors concluded that MMC
and other antimetabolites have a direct toxic effect on the ciliary body
epithelium, besides their known effect on the conjunctiva. The application of
MMC, both topically in glaucoma filtering surgeries and by the subconjunctival
method of Mahar et al in glaucoma patients,13 has yielded significant decreases in IOP in
both experimental and human models. Since topical MMC is extensively used as an
adjunct in pterygium excision to prevent recurrence, the purpose of this study
was to determine the effect of MMC on ciliary body epithelium through the changes
in IOP in eyes that were undergoing pterygium excision with topical MMC.
MATERIAL AND METHODS
This non-randomized interventional case series was performed at
the Section of Ophthalmology, Department of Surgery, Aga Khan University
Hospital, Karachi, Pakistan, from 2005 to 2010. One hundred and fifty six
patients with unilateral progressive pterygium who had undergone supervised
surgical excision by the bare sclera technique with MMC were enrolled. The
exclusion criteria were previous drainage surgery, suspicious growth other than
pterygia or corneal scarring, antiglaucoma therapy in either eye, history of
Sjogren’s syndrome or any other ocular disease, and keratoconjunctivitis sicca.
The study protocol was approved by the Hospital Ethics Committee and the study
was performed in accordance with the Declaration of Helsinki. All patients
provided informed consent. The primary outcome measure was to determine the
toxic effect of MMC on the ciliary body epithelium through the comparison of
mean baseline IOP with the IOP measured in the ipsilateral eye affected by
pterygium at 3 months after intraoperative treatment with topical MMC.
The baseline IOP measurement was established by taking the mean of
the two highest values measured at 9:00 am and 4:00 pm by Goldmann applanation
tonometry (GAT) before pterygium excision. All patients underwent complete
ocular exami-nation, including best-corrected visual acuity, biomi-croscopic
examination of the anterior segment with GAT, and fundus examination with a +90
diopter lens.
Pterygium excisions
were performed on an outpatient basis by the same surgeon (PSM) using the same
technique.14 No premedication was
given to any patient. After pterygium excision with the bare scleral technique
under topical anesthesia (Proparacaine, Alcon – Belgium), a 5- x 5-mm sterile
sponge soaked in 8 to 10 drops of MMC (Kyowa – Japan) 0.2 mg/mL19–21
was applied over the corneosclera and the area from where pterygia was excised
for 3 minutes. The sponge was removed and the eye was irrigated with 20 ml of
0.9% normal saline. This was followed by topical administration of
dexamethasone 0.1% plus tobramycin 0.3% (Tobradex, Alcon – Belgium) and
hydroxypropyl methylcellulose (Tear Naturale II, Alcon – Belgium), which was
instilled 4 times daily for 4 weeks to prevent postoperative inflammation. The
patients’ IOPs were measured on days 1, day 7 at 1 month and after 3 months.
Any adverse effects or physical findings were also noted at each visit.
Statistical Analysis
The data analysis
was conducted into the statistical package for the social sciences version 16
(SPSS Inc. Chicago, USA). The entire continuous variable i.e. age, baseline
IOP, post-op IOP and change in IOP presented as mean ± standard deviation and
categorical variables like gender, affected eye, IOP and pterygium site
presented as frequency and percentage. To estimate the comparison between the
IOP’s, we applied paired sample t test using preoperative levels. The IOP was
considered to be higher or lower than the preoperative level if the difference
was more than 5 mm Hg. The IOP value measured preoperatively was taken as the
baseline measurement to reduce any bias due to recruitment.
RESULTS
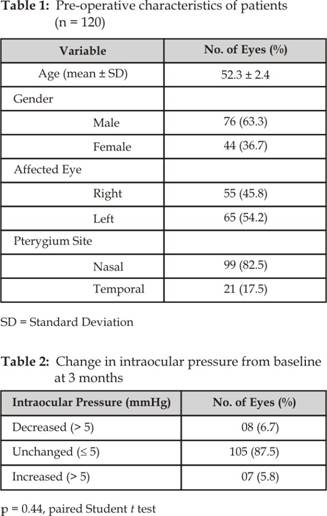
One hundred and fifty six patients were enrolled; 120 eyes of 120
patients were followed for at least 3 months, 36 patients were lost to
follow-up and hence their data has been excluded from this study. There were 76
male (63.3%) and 44 female (36.7%) with a mean age of 52.3 years (range, 26 to
83 years) and standard deviation 2.4. The pterygium was located on the nasal
side in 99 eyes (82.5%) and on the temporal side in 21 eyes (17.5%). There were
55 right eyes and 65 left eyes. The baseline characteristics of the patients
are shown in (Table 1). There were no significant changes in IOP in 105 eyes
(87.5%) at 3 months (p = 0.44, paired Student t test); Eight eyes (6.7%)
had a decrease in IOP >5 mm Hg and 7 eyes (5.8%) had an increase in IOP
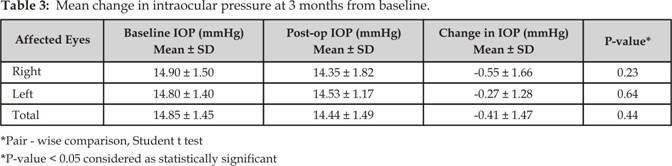
>5 mm Hg, which were not statistically significant (Tables 2 and 3).
Fifty five affected
eyes were on the right side, of which 49 eyes (89.1%) had no significant change
in IOP throughout the follow-up period (p = 0.23); 17 eyes (30.9%) had no
change in IOP and 31 (56.4%) had minimal changes (≤ 5 mm Hg). Three eyes
(5.4%) had a decrease in IOP of > 5 mm Hg and 4 (7.3%) had an increase in
IOP of > 5 mm Hg. There was a change in IOP level from a mean of 14.90 mm Hg
± 1.5 SD at baseline to a mean of 14.35 mm Hg ± 1.8 SD after 3 months, which
was statistically not significant.
Sixty five affected
eyes were on the left side, of which 56 eyes (86.1%) had no significant change
in IOP throughout the follow-up period (p = 0.64); 21
eyes (32.3%) had no change in IOP and 36 eyes (55.4%) had minimal changes
(≤ 5 mm Hg). Five eyes (7.7%) had a decrease in IOP of > 5 mm Hg and 3
(4.6%) had an increase in IOP of > 5 mm Hg. There was a change in IOP level
from a mean of 14.80 mm Hg ± 1.4 SD at baseline to a mean of 14.53 mm Hg ± 1.1
SD after 3 months, which was statistically not significant.
DISCUSSION
This study investigated the toxic effect of MMC on ciliary body
epithelium through the changes in IOP in eyes, undergoing pterygium excision
with topical MMC. In a laboratory study by Letchinger et al15,
subconjunctival injection of MMC was administered to rabbit eyes and a
consequent drop in IOP was noted. In an experimental study in monkeys, Kee et
al noted a decrease in IOP from baseline after administration of MMC, and a
possible mechanism of aqueous suppression was suggested to be responsible for
the IOP reduction16. In a clinical study by Gandolfi et al,17
subconjunctival injection of MMC was administered to 12 eyes with no perception
of light and a decrease of about 5 mm Hg (SD, 1.61 mm Hg) in IOP was observed
at 60 days. These researchers also performed tonography on their patients to
detect the possible effect of MMC on the aqueous outflow from the eye, and
found no significant change in the ‘C’ coefficient throughout the follow-up
period.
The results of this study differ from the results of the above
mentioned studies,15-17 in that the decrease in IOP was observed
only in 4% to 5% of patients, which is statistically insignificant. In a
prospective study, Raiskup et al described the long-term effect of
intraoperative application of MMC 0.2 mg/mL for 5 minutes in patients
undergoing pterygium excision and noted a normal IOP on follow-up.18
Similarly in a study by Mahar et al. patients undergoing pterygium excision
with MMC applied topically in 5 different group of patients with application
time difference of 1 to 5 minutes, no change in IOP greater than 5 mmHg was
seen in either of the groups19.