Retinopathy of
prematurity (ROP) is responsible for blindness in an estimated 50,000 children
in the world each year. In middle income countries 15 – 35% of childhood
blindness is due to ROP1,2. In the USA between 1999 – 2012, 13 – 14%
of childhood blindness was attributed to ROP3. Studies have shown
that this can amount to a financial burden of $69-117 million a year. These
estimates do not include loss of potential life long earnings, especially in
the developing countries where services to train individuals with blindness are
lacking. Although gestational age is the most important risk factor in the
development of ROP, there are other factors that have been implicated, such as
oxygen therapy.
In developed countries,
the 1940s-1950s saw the first epidemic of ROP due to inadequately monitored
oxygen therapy4-6. With changes in clinical practice, and controlled
oxygen administration, this epidemic was brought under control. In USA, the proportion
of blindness due to retrolental fibroplasia dropped from 50% in 1950 to 4% by
19657. However, the decrease in oxygen therapy resulted in an
increase in neonatal deaths, due to respiratory compromise8.
Increased survival rates of extremely premature (gestational age < 29 weeks)
and very low birth weight infants (750-999g) gave rise to the second epidemic
of ROP in the late 1970s and 1980s9-11. Data from the developing
countries is very limited. Gilbert et al, speculate that infant mortality rates
(IMR) may negatively correlate with the risk of ROP related blindness. With
improvement in neonatal care, more preterm infants are surviving worldwide; in
high income countries, with IMR of < 9 per 1000 live births, the risk of ROP
related blindness is low, due to good screening and treatment facilities. In
countries with high IMR (>60 per 1000 live births) not many preterm babies
survive, due to lack of basic health care facilities and proper neonatal
intensive care, so ROP is not a significant problem. However, middle income
countries in Latin America, Eastern Europe, India, China and other countries in
Asia, with IMR of 9 – 60 per 1000 live births, represent the population at the
highest risk of ROP blindness since 1990s. This has been described as the third
epidemic of ROP; although neonatal care has improved, good screening and
treatment facilities are inadequate in these regions1,2. Babies are
being exposed to risk factors which, to a large extent, have been addressed in
high income countries e.g., oxygen exposure.
Screening for the disease is a key component of the
treatment of ROP. Specific ‘standard’
criteria, based on gestational age of less than 32 weeks and birth weight of
less than 1500 gms, is being used in the United States. Results show, that 66% of infants < 1,250g
and 82% of infants < 1,000g developed ROP, while 9% became eligible for
treatment1-12. However, these screening criteria may not be
applicable to middle and low income countries, where more mature and heavier
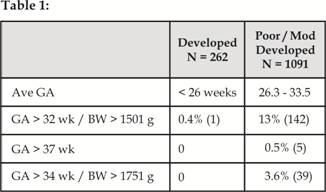
babies have been shown to develop ROP13-20. Gilbert et al
highlighted (Table 1) that 13% of infants would have been missed if the
‘standard’ screening criteria had been applied in such countries2.
Multiple
trials involving infants with ROP have highlighted the importance of timely
treatment to reduce the risk of blindness21-22. The latest trial
ETROP (Early Treatment for ROP) has shown that laser retinopexy within 48 hours
for type 1 ROP (definition) was associated with a decrease (19.8% to 14.3%) in
unfavorable visual outcomes23. Therefore screening protocols are
being followed in NICUs to identify infants needing treatment. Oxygen
regulation trials, such as STOP – ROP, SUPPORT and BOOST – II, have been
conducted to observe ophthalmic outcomes with supplemental oxygen13-15
and the results reveal that ROP is best controlled by avoidance of fluctuations
and by strict maintenance of SpO2 between 85% - 92% in theses babies24,25.
ROP
IN PAKISTAN
Neonatal care services have expanded and
more premature babies are now surviving. Infant mortality rate (IMR) in
Pakistan dropped to 61.3 per 1000 live births in 2012 from 82.5 per live births
in 2000, thus Pakistan is now at the threshold for an epidemic of blindness due
to ROP26.
There are only 2
published studies on ROP, both from Aga Khan University Hospital, with a very
well equipped tertiary care NICU. In 2008, a retrospective analysis of 68
premature infants with birth weight < 1500 gm and gestational age
< 32 weeks, had reported an incidence of 32.4% of any stage of ROP, with
20.6% with severe ROP27. A later studied conducted prospectively at
the same institute with a broader screening criterion i.e., birth weight
≤ 2000g and gestational age ≤ 35 weeks – any stage of ROP, showed
that no ROP was seen in the 66/301 infants who weighed > 1500g at birth
and/or were born at > 32 weeks of gestation. Using the standard
screening criteria, there was an improvement with only 11.5% developing ROP,
while stage 3 ROP requiring treatment were 8.1% of the cases as compared 20.6%,
in the earlier study28. These levels are now comparable to ROP
outcomes in high income countries.
There
is still a severe lack of awareness of the disease, appropriate screening
criteria, consequences of delayed or no treatment as well as a lack of
expertise for the management of such babies29. In 2010 a descriptive
study conducted at 10 centers with highest delivery rates in Karachi showed
that only 2 centers had a screening protocol for ROP in place, but which was
not being followed. Only 2 out of the 15 pediatricians who were interviewed
were aware that ROP can cause blindness30.
FUTURE
RECOMMENDATIONS
There is an urgent need for creation of
appropriate screening and oxygen protocols, training of ophthalmologist to
screen the infants, creation of close liaison between the NICU, ophthalmologist
and parents, education for all care givers on the importance of ROP, to protect
premature infants in Pakistan from permanent blindness.
We propose the creation of a Pakistan
Retinopathy of Prematurity Educational
and Research Alliance (PROPERA). Initially, a few
hospitals in 2 to 3 major cities should be involved. An ROP coordinator and an
ophthalmologist for screening should be designated. An initial screening
criteria and an Oxygen protocol should be followed at these sites. Data should
be collected and transmitted to a central collection center weekly.
Subsequently, the network should be expanded, by adding other centers, and
additional cities. An annual ROP conference should be organized to include all
health care individuals involved with management of infants at risk of ROP.
Collected ROP data should be presented, deficiencies identified, creation of
appropriate screening guidelines and formulation of a plan for the next year
agreed upon.
Individuals
with experience and interest in ROP will be vital for the success of this
endeavor to save the sight of our next generation.
Author’s Affiliation
Dr. Umar K. Mian
Director Retina Service
Department of Ophthalmology and Visual
Sciences
Montefiore Medical Center / Albert Einstein
College of Medicine, New York, USA
FOR PROPERA
NETWORK
Dr. Farzeen Hashmi, Dr. Tanveer Chaudhry
Dr. Khabir Ahmad
Department of ophthalmology
Aga Khan University Hospital, Karachi
REFERENCES
1. Gilbert, C. Retinopathy of prematurity: a global perspective of the
epidemics, population of babies at risk and implications for control. Early
human development. 2008; 84: 77-82.
2. Gilbert C, et al. Characteristics of infants with severe
retinopathy of prematurity in countries with low, moderate, and high levels of
development: implications for screening programs. Pediatrics. 2005;. 115:
518-25.
3. Kong L, et al. An update on progress and the changing epidemiology
of causes of childhood blindness worldwide. Jr of Am Assoc. for Pediatric
Ophthalmology and Strabismus. 2012; 16: 501-7.
4. Campbell K. Intensive oxygen therapy as a possible cause of
retrolental fibroplasia; a clinical approach. The Medical J of Australia. 1951;
2: 48.
5. Patz A, Hoeck LE, Cruz EDL. Studies on the effect of high oxygen
administration in retrolental fibroplasia. I. Nursery observations. Am J Ophthalmol.
1952;. 35: 1248.
6. Ashton, N., B. Ward, and G. Serpell, Effect of oxygen on developing
retinal vessels with particular reference to the problem of retrolental
fibroplasia. The Br J Ophthalmol. 1954; 38: 397.
7. Hatfield EM. Blindness in
infants and young children. Sight-Saving Review. 1972.
8. Avery ME, EH. Oppenheimer, Recent increase in mortality from
byaline membrane disease. The Jr of Pediatrics, 1960; 57: 553-9.
9. Gibson, DL, et al. Retinopathy of prematurity: a new epidemic?
Pediatrics. 1989; 83: 486-92.
10. Valentine PH, et al. Increased survival of low birth weight
infants: impact on the incidence of retinopathy of prematurity. Pediatrics.
1989; 84: 442-5.
11. Todd DA, et al. Retinopathy of prematurity in infants less than 29
weeks' gestation at birth. Australian and New Zealand J of Ophthalmol. 1994;
22: 19-23.
12. Zin A,. Gole GA. Retinopathy of
prematurity-incidence today. Clin Perinatol. 2013; 40; 185-200.
13. Jalali S, et al.
Modification of screening criteria for retinopathy of prematurity in India and
other middle-income countries. Am J Ophthalmol. 2006, 141: 966-8.
14. Vinekar A, et al.
Retinopathy of prematurity in Asian Indian babies weighing greater than 1250
grams at birth: ten year data from a tertiary care center in a developing
country. Indian J Ophthalmol. 2007; 55: 331-6.
15. Chen Y, Li X.
Characteristics of severe retinopathy of prematurity patients in China: a
repeat of the first epidemic? Br J Ophthalmol. 2006; 90: 268-71.
16. Filho FJB, et al.,
Results of a program for the prevention of blindness caused by retinopathy of
prematurity in southern Brazil. Jr Pediatr (Rio J). 2007; 83: 209-16.
17. Zin AA,, et al.
Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening
criteria and workload implications. Pediatrics. 2010, 126: 410-7.
18. Araz-Ersan B, et al.
Epidemiological analysis of retinopathy of prematurity in a referral centre in
Turkey. Br J Ophthalmol. 2012.
19. Binkhathlan AA, et al.
Retinopathy of prematurity in Saudi Arabia: incidence, risk factors, and the
applicability of current screening criteria. Br J Ophthalmol. 2008; 92: 167-9.
20. Amer M, et al.
Retinopathy of prematurity: are we missing any infant with retinopathy of
prematurity? Br J Ophthalmol. 2012; 96: 1052-5.
21. Mintz-Hittner HA, Kennedy
KA, Chuang AZ. Efficacy of
intravitreal bevacizumab for stage 3+ retinopathy of prematurity. New England J
of Medicine. 2011; 364: 603-15.
22. Cryotherapy for Retinopathy of Prematurity Cooperative, G.,
Multicenter trial of cryotherapy for retinopathy of prematurity:
ophthalmological outcomes at 10 years. Archives of Ophthalmology. 2001. 119:
1110.
23. Good WV. Final results
of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial.
Transactions of the American Ophthalmological Society. 2004; 102: 233.
24. Saugstad OD. Oxygen and
retinopathy of prematurity. Jr of Perinatology. 2006; 26: 46-50.
25. Chow LC, Wright KW, Sola A.
Can changes in clinical practice decrease the incidence of severe retinopathy
of prematurity in very low birth weight infants? Pediatrics, 2003; 111: 339-45.
26. Khan A, et al. Newborn
survival in Pakistan: a decade of change and future implications. Health Policy
and Planning. 2012; 27: 72-87.
27. Taqui AM, et al.
Retinopathy of prematurity: frequency and risk factors in a tertiary care
hospital in Karachi, Pakistan. Jr Pak Med Assoc. 2008; 58: 186-90.
28. Chaudhry TA, et al.
Retinopathy of prematurity: an evaluation of existing screening criteria in
Pakistan. Br J of Ophthalmol. 2013; 30: 4018.
29. Sethi S, Awan H, Khan NU. An
audit of Neonatal Services in Khyber Pakhtunkhwa Province (KPK), Pakistan to
identify Implications for screening ‘Retinopathy of Prematurity’. Ophthalmology.
2012; 10: 136-42.
30. Hashmi FK, Chaudhry TA, Ahmad
K. An evaluation of referral system for retinopathy of prematurity in
leading health centers across Karachi, Pakistan. Jr Pak Medical Assoc. 2010;
60: 840.