Original Article
Prevalence of Diabetic
Retinopathy among Type – 2 Diabetes Patients in Pakistan – Vision Registry
Mehreen Sohail
Pak J Ophthalmol 2014, Vol. 30 No. 4
. . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . ..
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
|
See end of article for authors affiliations …..……………………….. Correspondence to: Mehreen Sohail 367, K, Phase V DHA, Lahore Email: mehreen61@gmail.com …..……………………….. |
Purpose: To
estimate the prevalence of diabetic retinopathy (DR) among patients with type
2 diabetes mellitus (T2DM) in Pakistan. Material and Methods: This is a cross-sectional study carried out in 25 centers across
Pakistan between July 2009 to May 2010. Each centre
recruited 9 consecutive patients meeting the eligibility criteria of age ≥ 18 years with known T2DM
for ≥ 3 years and willing to provide written
consent. Direct ophthalamoscopy to determine DR and
blood tests for random blood sugar (RBS) and HbA1c levels, were
conducted. Descriptive statistics (frequency, proportion, and mean) were used
to analyze the data. Results: Of
the 223 patients recruited, analysis was based on data gathered from 202
patients. The mean age of the patients was 52.9 ± 10.5 years, and their
average RBS and HbA1c levels were 219.2 ± 82.4 mg/dL and 8.9 ± 2.5%, respectively. Mean duration of
diabetes was 8.8 ± 5.1 years. Over three-fourths (77.2%) of the patients had
never been assessed for DR. The prevalence of DR was calculated at 56.9%
(confidence interval: 50.1 – 63.3%). Factors associated with DR were systolic
blood pressure (p = 0.009), diastolic blood pressure (p = 0.001)
and duration of diabetes (p = 0.04). Conclusions: The
prevalence of DR in Pakistan is substantially high. Regular screening needs
to be implemented for early diagnosis of DR. Key
Words: Diabetic Retinopathy,
Prevalence, Type 2 diabetes mellitus. |
Diabetes mellitus is a non-communicable medical disorder
characterized by hypergly-caemia due to defective
insulin secretion and is currently amongst the top ten causes of worldwide
mortality.1 The incidence of diabetes is on the rise, especially in
developing nations like India and China,2,3 and the estimated global
burden for the year 2030 is 439 million people.2 Pakistan currently
ranks sixth amongst countries with the highest number of diabetes patients, and
more than 11% of Pakistani adults have diabetes.4 It is predicted
that by 2030, Pakistan will rise to the 5th position with 13.9
million diabetic patients.5
Chronic hyperglycaemia in diabetes leads
to various macrovascular (coronary heart disease,
peripheral vascular disease, and stroke) and microvascular
(retinopathy, neuropathy, and nephropathy) complications.6 Given the
observation that diabetes in most patients is diagnosed late, these micro- and macrovascular complications are already present in the
patients at the time of diagnosis, and the frequency of their coexistence
increases with the duration of diabetes.7
Diabetic retinopathy (DR) is the leading cause of visual
impairment in adults worldwide8. In DR, the
blood vessels in the eye become swollen and leaky and new abnormal vessels form
on the retina. Eventually, DR causes irreversible blindness9.
According to the American Diabetes Association (ADA), 21% of patients with
diabetes have DR at diagnosis10 and more than 60% of patients with
diabetes will have DR within two decades of diagnosis.11 A recent
meta-analysis of 35 population-based prevalence studies carried out in the US,
Europe, Australia and Asia over a period of 28 years with data from 22,896
diabetes patients, revealed that the overall prevalence of DR is as high as
34.6% and more than 10% of the diabetes patients have vision – threatening DR.12
The findings of the two major diabetes trials, the Diabetes
Control and Complications Trial 13 and the United Kingdom
Prospective Diabetes Study,14 have established the importance of
tight glycaemic control (target HbA1c
levels under 7%) in reducing the risk of microvascular
complications. This is especially beneficial in the early stages of DR and
nephropathy. However, a vast majority of patients who develop DR do not display
any symptoms till late stage. Since, early detection can prove beneficial in
symptomatic amelioration and slowing the progression of DR, it is important to
screen patients with diabetes for retinal disease on a regular basis15.
According to ADA guidelines, ophthalmic examination should be conducted at the
time of diabetes diagnosis16, and repeated annually unless it is the
ophthalmologist’s clinical judgment to have the exam every 2 – 3 years.17
In Pakistan, there is insufficient data on the national prevalence
and management of DR. A few community or hospital or region-based studies have
been conducted, but the reported DR prevalence rates vary widely (15% – 33.3%).18-22
It is also estimated that only about 33% to 44% of the
patients with diabetes in Pakistan have accurate knowledge of their disease and
its complications.4,23
Cross-sectional studies play a vital role in determining the extent of the
disease prevalence and can aid in implementation of effective strategies for
early diagnosis, management, and patient education / awareness.
Accordingly, we present the findings of the Prevalence of Diabetic Retinopathy
amongst type – 2 diabetic population in Pakistan
(VISION) registry that was designed to assess the prevalence of DR among
diabetes patients in Pakistan and the association between DR and glycaemic control.
MATERIAL
AND METHODS
The VISION registry was a national, multicentre,
non-interventional, cross-sectional registry. It was designed to primarily estimate
the prevalence of DR amongst patients with type 2 diabetes in Pakistan. The
secondary objectives of this study were to 1) determine the distribution of DR
across HbA1c levels; 2) document patient profile of all patients
willing to participate; and 3) document other diabetic complications based on
clinical signs and symptoms and / or historical evidence. The study was
conducted in 25 randomly selected centres from 9
cities across 4 provinces in Pakistan. The study was conducted in accordance
with the principles laid by the 18th World Medical Assembly, the
guidelines of Good Epidemiology Practice and all local laws and regulations.
Written informed consent was obtained by the investigator from each patient
enrolled in the study.
Study investigators were selected from a list of qualified general
practitioners. Each centre was supported by services of qualified
ophthalmologists. Each investigator recruited 9 consecutive patients who met
the inclusion / exclusion criteria. Patients enrolled were of either gender,
aged ≥ 18 years with type 2 diabetes for ≥ 3 years, provided an
informed consent, and were willing to undergo ophthalmoscopic
examination. Patients with known ophthalmic disorders other than DR were
excluded.
On a scheduled day in the general practitioner’s clinic, study
patients were examined for evidence of DR by nine ophthalmologists. Fundoscopic examinations were conducted on dilated pupils
using a direct ophthalmoscope (Welch Allyn Inc, Skaneateles Falls, NY, USA). Random blood sugar (RBS)
levels were measured using OneTouch® blood glucose meter (Life Scan
Inc., a Johnson & Johnson Company, Milpitas, CA). Diabetic neuropathy was
determined by 10-g Semmes-Weinstein monofilament examination. Additionally, 2
consecutive seated blood pressure readings were recorded at 3 minutes interval.
Patients also underwent the HbA1c test by NGSP certified HbA1c
machine at a central laboratory (The Aga Khan University Hospital, Karachi).
Patient data was recorded on case report forms and included
details on general and lifestyle information, diabetic history, RBS and HbA1c
levels, blood pressure, anthropometric measurements, ophthalmoscopic
and microfilament findings and history of nephropathy, if present. Patients
with DR findings were referred to specialized eye care centres
for further consultation.
Given a reported prevalence of 26% of DR amongst a DM prevalence
of 11% in Pakistan24, 225 patients were planned to be recruited to
give the study a precision of ± 6% at 95% confidence interval (CI) after
accounting for incomplete forms, withdrawal after consent, etc.
Being a descriptive cross-sectional study, categorical variables
are reported as proportions and percentages while continuous variables are
reported as mean with standard deviation (SD).
RESULTS
Of the 223 patients recruited, analysis was based on data gathered
from 202 patients. The average age of patients evaluated was 52.9 ± 10.5 years
(Table 1). There were more men (53.5%) than women (46.0%) enrolled. Average
body mass index (BMI) was 28.6 ± 8.9 and the mean duration of diabetes in the
patients was 8.8 ± 5.1 years. The average blood pressure was 133.5 ± 17.4 mm Hg
systolic and 86.1 ± 9.6 mm Hg diastolic. Mean RBS was 219.2 ± 82.4 mg/dL while average HbA1c was 8.9 ± 2.5%.
The most commonly observed risk factor was hypertension, reported
in 125 (61.9%) patients, followed by sedentary lifestyle, reported in 90
(44.6%) patients (Table 1). Other risk factors reported in more than 20% of the
patients included metabolic syndrome, past smoking, and family history of
cardiovascular disorders.
Over three-fourths of the patients (n=163, 80.7%) were on oral antidiabetic (OAD) therapy (Table 1). The drug classes of
choice were biguanide (76.7%) and sulphonylurea
(74.8%). Only about one-third of the patients (35.6%) were on a single OAD
agent while half the number of patients (50.0%) were
on 2 OADs. Insulin monotherapy was reported in 4
(1.9%) patients while insulin in combination with OAD had been prescribed to 29
(14.4%) patients.
A total of 115 patients out of 202 (56.9%, CI: 50.1%-63.3%) had
DR. As shown in Figure 1, the most common DR findings were haemorrhages
(70/202, 34.7%), hard exudates (67/202, 33.2%), cotton wool spots (21/202,
10.4%) and neovascularization (15/202, 7.4%). A substantial number of patients (n
= 157, 77.7%) had never been assessed for DR prior to enrolment in the reported
study.
On 10-g monofilament examination, neuropathy was detected in 59.9%
(121/201) patients and nephropathy was reported by 6.4% (13/202) patients.
A comparison of various parameters in patients with and without DR
is presented in Table 2. Patients with DR had a higher systolic blood pressure
than patients without DR (136.4 ± 17.9 mmHg versus 129.7
± 16.0 mmHg; p = 0.009). Similarly, diastolic blood pressure in patients with
DR was higher than patients without DR (88.1 ± 9.8 mmHg versus 83.5 ± 8.7 mm
Hg; p = 0.001).
Moreover, patients with DR had had DM for
a longer period than those without DR (average duration 9.4 ± 5.6 versus 7.9 ± 4.2
years, p = 0.04). There was no statistically significant difference in the
association of DR with other risk factors. In addition, more percentage of
patients without than with DR were on OAD monotherapy
(34.9% versus 17.4%; p = 0.005).
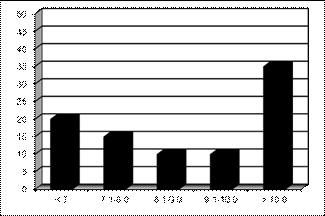
Diabetic retinopathy was prevalent across all levels of HbA1c
values (Figure 2). The highest prevalence of DR was in patients with HbA1c
levels > 10% (41/115, 35.6%). Interestingly, the group with the next –
highest prevalence was the one with HbA1c levels < 7% where 45 (22.3%)
patients had DR.
DISCUSSION
With a burgeoning epidemic of diabetes in South Asia and the
significant impact of diabetic complications on patients and the healthcare
system, the VISION registry aimed at estimating the prevalence of DR in
Pakistan. The findings of this first attempt at understanding the pervasiveness
of DR nationally did reveal some very significant results.
In
comparison to the previously reported DR prevalence of 26% in patients with
diabetes by Khan et al in 1991,24 the current prevalence has doubled to 56.9%,
which is substantially higher than any previously reported value worldwide12.
While our study was not designed to identify the reasons for this dramatic
increase, one can only speculate on subjective factors like lack of patient and
physician education, glycaemic control, treatment
adherence, and regular screening for DR. The latter holds especially true since
we discovered that despite Fig. 2: Distribution
of patients with diabetic retinopathy by their HbA1c levels, N = 115
having diagnosed diabetes for an average duration of 8.8 ± 5.1 years, over
three-fourths of the patients had never been assessed for presence of DR prior
to enrolment in the VISION registry. Since DR progression can be slowed with
early detection, this finding provides impetus to include retinal screening as
a routine part of diabetes management, and general practitioners need to have a
baseline assessment of their diabetic patients upon diagnosis. Moreover,
comprehensive patient education programs on DR should be provided by the physician/ophthalmologist
at the time of diagnosis of diabetes.


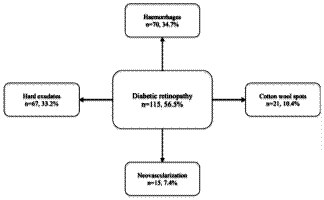
Fig. 1: The most common diabetic
retinopathy findings noted in analysed patients
(n=115)
A/ % of patients
HbA1c Levels
![]()
Data was missing for 9
patients
Data was missing for 5
patients
The other major finding of the VISION registry is that it revealed
the association of elevated blood pressure with DR. The systolic blood pressure
in patients with DR was higher than that in patients without DR (136.4 ± 17.9
mm Hg versus 129.7 ± 16.0 mm Hg; p=0.009). Also, the diastolic blood pressure
in patients with DR was higher than that in patients without DR (88.1 ± 9.8 mm
Hg versus 83.5 ± 8.7 mm Hg; p = 0.001). We also discovered that
average duration of diabetes was longer in patients with DR (9.4 ± 5.6 versus
7.9 ± 4.2 years; p=0.04) than that in patients without DR. The correlation between
blood pressure and duration of diabetes with DR has been demonstrated in recent
studies and our findings reiterate these.25-27
Hypertension and diabetes are usually co-morbid. Patients with
diabetes are 1.5 – 2 times more susceptible to hypertension than patients
without diabetes28 and the co-existence of diabetes and hypertension
is shown to accelerate microvascular complications29.
A recent study to estimate the global prevalence of DR indicated hypertension
as one of the major risk factors for DR.12 In the
VISION registry, we observed that the most common risk factor was hypertension,
reported in 61.9% patients. The proportion of patients with hypertension was
almost the same in patients with or without DR (64.3% vs. 60.7%, p = 0.8) However,
patients with DR were relatively inadequately controlled for blood pressure
compared to those without DR as described above. Better control of blood
pressure in diabetic patients is likely to help impede the progression of DR.
Acetylated haemoglobin (HbA1c)
level is another major indicator of risk for DR. Diabetic patients with a tight
glycaemic control of HbA1c < 7% have
slower progress of microvascular complications while
those with poor glycaemic control tend to rapidly
deteriorate.12 The
other major observation from the landmark Diabetes Control and Complication
Trial is that even after regaining appropriate glycaemic
control, a prolonged preceding hyperglycaemia does
not halt the progression of DR.30 This imprinted effect of high
blood glucose even after normal levels have been attained is termed as
“metabolic memory” and plays an important part in the development and
progression of diabetic complications, especially DR.31-33 The
VISION registry revealed that patients with DR were present across the range of
HbA1c levels. Expectedly, the rate of prevalence of DR (35.6%) was
highest in patients whose HbA1c levels were above 10%. However, the
group with the next highest DR prevalence rate was the one in which the mean
HbA1c levels were < 7%. While this does not conform to the
observations from other studies34-35 could probably be attributed to the presence of other contributing risk
factors – hypertension, peripheral artery disease, etc. It may also be
postulated that these patients to begin with had an elevated HbA1c
and also developed DR but eventually managed to have a better glycaemic control without reversal of DR changes. This
suggests that early diagnosis and good glycaemic
control at initial stage of diabetes sets in a good metabolic memory and hence
are critical in preventing or delaying onset of DR. Considering the limitation
of cross sectional study it is suggested to follow the temporality of
observations in such cohort of patients. Nonetheless, one can advocate early
detection through regular blood check-ups and achievement of tight glycaemic control for delaying the progression of DR.
The other clinically significant complications of diabetes are
neuropathy and nephropathy. Diabetic neuropathy usually results in foot
ulceration, Charcot neuroarthropathy, and limb
amputation;36 while diabetic nephropathy leads to chronic renal
failure.37 Though there is a dearth of information on the global
prevalence of these complications, certain regional studies indicate that the
prevalence of neuropathy is between 22% and 29% amongst the diabetics in
Europe,38-40 and the prevalence of nephropathy is 5.5% in India and
22.3% in Asian Indians in the United Kingdom.41 Given the
seriousness of these diabetic complications it is equally necessary to monitor
the prevalence of these in patients with diabetes.42 In the VISION
registry, a total of 6.4% of the diabetics had comorbid nephropathy. However,
the prevalence of neuropathy was at a staggering 59.9%. This finding raises
some critical questions on whether we are doing enough to increase awareness
amongst patients and physicians, to ensure our physicians are compliant with
international guidelines, to understand the gap between real-world practices
and international recommendations, and to estimate the prevailing load of
diabetic complications in our country. Once understood, we can implement
effective strategies to positively influence public health and decrease the
economic burden of diabetes in Pakistan.
Another observation from our study was the pharmacotherapy of Type
2 diabetes in Pakistan. More than 80% of the patients were prescribed OAD, a
substantial number (n = 101; 50.0%) of these being prescribed a dual therapy,
usually biguanide and sulphonylurea.
Insulin usage was reported in a bit over 15% of the study patients. This is not
entirely surprising given the ease of administration of OADs. Besides, most
physicians and patients are hesitant to initiate insulin treatment due to the
fear of injectable drug delivery, hypoglycaemia,
weight gain and a “psychological insulin resistance”.43-44
Traditionally, management of diabetes progresses from lifestyle management to
OAD to insulin.45 However, keystone studies have demonstrated that
insulin therapy reduces micro- and macrovascular
complications in diabetics.46,47 Currently, a new school of thought
is emerging with its premise being early insulinization
to elicit long-lasting glycaemic control.45
In support, recent clinical trials have demonstrated the benefits of insulin
therapy in new Type 2 diabetics in terms of glycaemic
control, treatment satisfaction and quality of life.48,49 The
observation that over half of the patients in our study had DR but were still
managed with OADs warrants the need for a well-monitored, better pharmacologic
management of Type 2 diabetes.
VISION registry provides seminal insights on the burden DR in
Pakistan despite few limitations. Being a cross-sectional study, it does not
reveal the reasons for the surge in the prevalence of DR in Pakistan within a
span of > 20 years. This apparent surge may yet be an underestimate of the
disease burden as this study was conducted in the offices of the general
practitioner, who is the primary contact for majority of the population. It is
also known that for every patient seeking care at the grass root level there is
at least an equal number who for different reason may not seek care.50
Moreover, the patients in this study were only examined for the presence of DR
and not classified for a particular kind or a particular stage of DR. Current
statistical analysis was simple descriptive addressing study objectives.
Rigorous data mining may generate more hypotheses for future perusal.
CONCLUSION
In conclusion, this first nationwide DR registry does indicate the
gravity of the situation in Pakistan and serves as a stimulus to overhaul the
current diabetes management practices and implement more appropriate and
contemporary initiatives.
ACKNOWLEDGEMENTS
We duly thank all the participating physicians from: 1) Lahore -
Dr Atif Bashir, Dr Khalid Mehmood,
Dr Iftikhar Hussain, and Dr Bakhtawar Ali; 2) Sukkur – Dr Maqsood Gul
Awan and Dr Rasheed Kumbho; 3) Hyderabad
– Dr Muhammad Irshad Ahmad,
Dr Idrees Bawani, and Dr Aziz ur Rehman; 4) Gujranwala Dr Haji Maqsood Mahmood; 5) Faisalabad - Dr Khalid Javed
and Dr Wasim Ahmad Tariq;
6) Multan - Dr Faiz Athar Khan and Dr Muhammad Safdar; 7) Rawalpindi - Dr Shehzad Tahir, Dr Ehsan ul
Haque, Dr Tahir Mehmood Mirza,
and Dr M Farooq Sheikh;
8) Peshawar – Dr Muhammad Asif Iqbal and Dr Muhammad Irfan; 9)
Karachi – Dr Aslam Pervaiz, Dr M. Shafqat Mirza, Dr Shaukat Ali, Dr Jabbir Hussain,
and Dr Faizullah Lokhandwala.
Dr. Amman Ullah Khan and Dr. Nabeea Junaid, for conducting
study design Iqbal Mujtaba from Sanofi (Pakistan)
for conducting statistical analysis. Satyendra Shenoy and Anahita
Gouri from Sanofi (India) for providing assistance in writing this manuscript.
The study was funded by Sanofi Pakistan Limited.
Author’s Affiliation
Dr. Mehreen Sohail
Consultant
Ophthalmologist,
Cavalry Hospital, Lahore
REFERENCES
1.
World Health Organization. The top 10 causes of
death.): World Health Organization, 2011.
2.
Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the
prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87:
4-14.
3.
Yang SH, Dou KF, Song WJ. Prevalence of diabetes
among men and women in China. N Engl J Med. 2010; 362: 2425-6.
4.
Shera AS, Rafique G, Khwaja IA, Baqai S, Khan IA, King H. Pakistan National Diabetes
Survey prevalence of glucose intolerance and associated factors in North West
at Frontier Province (NWFP) of Pakistan. J Pak Med Assoc. 1999;49:206-11.
5.
Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of
diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care.
2004; 27: 1047-53.
6.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull
CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and
microvascular complications of type 2 diabetes (UKPDS 35): prospective
observational study. Bmj 2000; 321: 405-12.
7.
McCulloch D. Overview of medical care in adults with diabetes mellitus.).
UpToDate: UpToDate, 2008.
8.
Klein BE. Overview of epidemiologic studies of diabetic retinopathy.
Ophthalmic Epidemiol. 2007; 14: 179-83.
9.
National Eye Institute.
Facts about diabetic retinopathy.): National Institutes of Health, 2012.
10. American Diabetes Association. Diabetic Retinopathy. Diabetes
Care. 2002; 25: S90-3.
11. Fong DS, Aiello LP, Ferris
FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care 2004; 27: 2540-53.
12. Yau JW, Rogers SL,
Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A,
Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R,
Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS,
Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L,
Yasuda M, Zhang X, Mitchell P, Wong TY. Global prevalence and major risk factors of diabetic
retinopathy. Diabetes Care 2012; 35: 556-64.
13. Lasker RD. The diabetes control and
complications trial. Implications for policy and practice. N Engl J Med 1993;
329: 1035-6.
15. Fraser C, D'Amico D. Prevention and treatment
of diabetic retinopathy.): Up To Date, 2008.
17. Executive summary: Standards of medical care in
diabetes--2010. Diabetes Care 2010; 33: S4-10.
18. Mahar PS, Awan MZ, Manzar
N, Memon MS. Prevalence of type-II diabetes mellitus and diabetic
retinopathy: the Gaddap study. J Coll Physicians Surg Pak; 20: 528-32.
19. Jamal u D, Qureshi MB,
Khan AJ, Khan MD, Ahmad K. Prevalence of diabetic retinopathy among individuals screened
positive for diabetes in five community - based eye camps in northern Karachi,
Pakistan. J Ayub Med Coll Abbottabad. 2006; 18: 40-3.
20. Afghani T, Qureshi N,
Chaudhry KS. Screening for diabetic retinopathy: a comparative study
between hospital and community based screening and between paying and non-paying
patients. J Ayub Med Coll Abbottabad. 2007; 19: 16-22.
21. Kayani H, Rehan N, Ullah
N.
Frequency of retinopathy among diabetics admitted in a teaching hospital of
Lahore. J Ayub Med Coll Abbottabad. 2003; 15: 53-6.
22. Wahab S, Mahmood N, Shaikh
Z, Kazmi WH. Frequency of retinopathy in newly diagnosed type 2 diabetes
patients. J Pak Med Assoc. 2008; 58: 557-61.
23. Jabbar A, Contractor Z,
Ebrahim MA, Mahmood K. Standard of knowledge about their disease among patients
with diabetes in Karachi, Pakistan. J Pak Med Assoc. 2001; 51: 216-8.
24. Khan AJ. Prevalence of diabetic
retinopathy in Pakistani subjects. A pilot study. J Pak Med Assoc. 1991; 41:
49-50.
25. Klein R, Knudtson MD, Lee
KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy:
XXII the twenty-five-year progression of retinopathy in persons with type 1
diabetes. Ophthalmology. 2008; 115: 1859-68.
26. Raman R, Gupta A,
Kulothungan V, Sharma T. Prevalence and risk factors of diabetic retinopathy in
subjects with suboptimal glycemic, blood pressure and lipid control. Sankara
Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study
(SN-DREAMS, Report 33). Curr Eye Res. 2012; 37: 513-23.
27. Zheng Y, Lamoureux EL,
Lavanya R, Wu R, Ikram MK, Wang JJ, Mitchell P, Cheung N, Aung T, Saw SM, Wong
TY. Prevalence
and Risk Factors of Diabetic Retinopathy in Migrant Indians in an Urbanized
Society in Asia: The Singapore Indian Eye Study. Ophthalmology. 2012.
28. Sahay B. API-ICP guidelines on
diabetes 2007. J Assoc Phys Ind. 2007; 55: 1-50.
29. Parving HH, Andersen AR,
Smidt UM, Christiansen JS, Oxenboll B, Svendsen PA. Diabetic nephropathy and
arterial hypertension. The effect of antihypertensive treatment.
Diabetes 1983; 32: 83-7.
30. White NH, Sun W, Cleary
PA, Danis RP, Davis MD, Hainsworth DP, Hubbard LD, Lachin JM, Nathan DM. Prolonged effect of
intensive therapy on the risk of retinopathy complications in patients with
type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications
Trial. Arch Ophthalmol. 2008; 126: 1707-15.
31. Roy S, Sala R, Cagliero E,
Lorenzi M.
Overexpression of fibronectin induced by diabetes or high glucose: phenomenon
with a memory. Proc Natl Acad Sci USA. 1990; 87: 404-8.
32. LeRoith D, Fonseca V,
Vinik A. Metabolic
memory in diabetes-focus on insulin. Diabetes Metab Res Rev 2005; 21: 85-90.
33. Kowluru RA, Zhong Q,
Kanwar M.
Metabolic memory and diabetic retinopathy: role of inflammatory mediators in
retinal pericytes. Exp Eye Res. 2010; 90: 617-23.
34. Cheng YJ, Gregg EW, Geiss
LS, Imperatore G, Williams DE, Zhang X, Albright AL, Cowie CC, Klein R,
Saaddine JB. Association of A1C and fasting plasma glucose levels with
diabetic retinopathy prevalence in the U.S. population: Implications for
diabetes diagnostic thresholds. Diabetes Care. 2009; 32: 2027-32.
35. Tapp RJ, Shaw JE, Harper
CA, de Courten MP, Balkau B, McCarty DJ, Taylor HR, Welborn TA, Zimmet PZ. The prevalence of and
factors associated with diabetic retinopathy in the Australian population.
Diabetes Care. 2003; 26: 1731-7.
36. Boulton AJ, Vileikyte L,
Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;
366: 1719-24.
37. Dabla PK. Renal function in
diabetic nephropathy. World J Diabetes. 2010; 1: 48-56.
38. Young MJ, Boulton AJ,
MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral
neuropathy in the United Kingdom hospital clinic population. Diabetologia.
1993; 36: 150-4.
39. Tesfaye S, Stevens LK,
Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, Nuber A, Pozza G,
Ward JD.
Prevalence of diabetic peripheral neuropathy and its relation to glycaemic
control and potential risk factors: the EURODIAB IDDM Complications Study.
Diabetologia. 1996; 39: 1377-84.
40. Cabezas-Cerrato J. The prevalence of
clinical diabetic polyneuropathy in Spain: a study in primary care and hospital
clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society
(SDS). Diabetologia. 1998; 41: 1263-9.
41. Ramachandran A. Epidemiology of diabetes
in India--three decades of research. J Assoc Physicians India. 2005; 53: 34-8.
42. Standards of medical care in diabetes--2012. Diabetes Care
2012; 35 Suppl 1: S11-63.
43. Nakar S, Yitzhaki G,
Rosenberg R, Vinker S. Transition to insulin in Type 2 diabetes: family physicians'
misconception of patients' fears contributes to existing barriers. J Diabetes
Complications. 2007; 21: 220-6.
44. Polonsky WH, Fisher L,
Guzman S, Villa-Caballero L, Edelman SV. Psychological insulin resistance in patients with type 2
diabetes: the scope of the problem. Diabetes Care. 2005; 28: 2543-5.
45. Swinnen SG, Hoekstra JB,
DeVries JH. Insulin therapy for type 2 diabetes. Diabetes Care. 2009;
32: S253-9.
47. Holman RR, Paul SK, Bethel
MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2
diabetes. N Engl J Med. 2008; 359: 1577-89.
48. Weng J, Li Y, Xu W, Shi L,
Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, Ran X, Luo Z, Xian J, Yan L, Li F,
Zeng L, Chen Y, Yang L, Yan S, Liu J, Li M, Fu Z, Cheng H. Effect of intensive
insulin therapy on beta-cell function and glycaemic control in patients with newly
diagnosed type 2 diabetes: a multicentre randomised parallel-group trial.
Lancet. 2008; 371: 1753-60.
49. Houlden R, Ross S, Harris
S, Yale JF, Sauriol L, Gerstein HC. Treatment satisfaction and quality of life using an early
insulinization strategy with insulin glargine compared to an adjusted oral
therapy in the management of Type 2 diabetes: the Canadian INSIGHT Study.
Diabetes Res Clin Pract. 2007; 78: 254-8.
50. Hart JT. Rule of halves:
implications of increasing diagnosis and reducing dropout for future workload
and prescribing costs in primary care. Br J Gen Pract. 1992; 42: 116-9.