Glaucoma is one of the most common causes
of permanent visual loss all around the world,1
affecting about 60 million people worldwide2. In Pakistan, it
is the fourth commonest cause of visual loss3. Glaucoma is
characterized by optic nerve degeneration causing visual field defects and is
usually associated with raised intraocular pressure (IOP)4.
The main aim of treatment is to lower the intraocular pressure to preserve the
vision and prevent progressive optic nerve degeneration5. Various
treatment options include medical therapy, laser and surgical treatment. Timolol a beta-blocker acts by decreasing aqueous
secretion. It has been found to be effective for treatment in all types of
glaucoma6. Dorzolamide causes
highly selective inhibition of carbonic anhydrase II isoenzyme
present on the ciliary processes in the eye which
lowers the aqueous humor production and intraocular pressure7. Dorzolamide and Timolol are also used
in combination with additive therapeutic effect. The efficacy of fixed
combination of Timolol and Dorzolamide
(FCDT) is well established. The clinical efficacy of (FCDT) defined as 20% or
more reduction in mean intraocular pressure was found to be 82.9% in a study conducted by Andrew et
al8. Travoprost, a prostaglandin analog is one of the newer
anti-glaucoma drugs. It can strongly lower intraocular pressure9.
The clinical efficacy of Travoprost, (defined as 20%
or more reduction in mean IOP) was found to be 58.8% in a study conducted by Yuya Nomura et al10.
Another study showed that 64.2% of the patients treated with Travoprost had marked reduction in intraocular pressure11.
Trovoprost offers better patient compliance as
it is given in once daily dose as opposed to twice daily dose for FCDT and hence
Trovoprost is emerging as an alternative to the FCDT12
due to cost effectiveness and better compliance. Studies comparing the clinical efficacy of Trovoprost
against the traditional FCDT combination are scarce. Moreover the clinical
efficacy of Trovoprost is yet to be evaluated in our
local population. Our study aims to assess the clinical efficacy of the newer
drug Travoprost 0.004% against the established
combination of Timolol and Dorzolamide
in our local population. On the basis of
this study, if Travoprost is found to be effective,
this can be prescribed in routine instead of Timolol
plus dorzolamide for patients with primary open angle
glaucoma, with better compliance and safety profile.
MATERIAL AND METHODS
Patients with open angle glaucoma were selected
from outpatient department, Department of Ophthalmology, Khyber Teaching
Hospital, Peshawar as per operational definition. The purpose and benefits of the study were explained to the patient and
the patient was explained that this research study is being done purely for
research and a written informed consent was obtained, if agreed upon. Patient
compliance was stressed upon by education of the patient, relatives and by
checking of the used bottles by patient. Patients with newly diagnosed primary
open angle glaucoma with either gender between the ages of 15 to 60 years were
included in the study. Exclusion criteria were: Patients with IOP > 30,
advanced visual field loss, CDR > 0.8 or best corrected visual acuity <
6/60 (these patients have advanced disease and require more aggressive
treatment and this may act as confounder and affect the results of the study),
patients in whom beta blockers are contraindicated e.g. patients with COPD,
asthma, sinus bradycardia, heart block, and patients using drugs which can affect the
intraocular pressure e.g. patients already on anti-glaucoma medications or on
systemic beta blockers. After inclusion
in the study, patients was divided into group A and group B by lottery method i,e first patient went either into
Group A or Group B by simple lottery and the subsequent patients were consectively placed in the respective groups. Group A received once
daily travoprost 0.004% and B received twice daily timolol 0.5%
plus dorzolamide 2% combination. In both groups, detailed history was
taken followed by complete examination including assessment of best corrected
visual acuity (BCVA) using Snellen chart; pupillary
reaction, anterior segment examination with slit-lamp; baseline IOP measurement
with Goldman applanation tonometer; anterior chamber
angle assessment with Goldman goniolens; fundus
examination with direct ophthalmoscope and 90 D lens and perimetry
(Humphrey’s). The patient was advised to come at 6 weeks interval for follow
up. At each follow up visit, IOP was recorded. Efficacy was defined as at least
20% reduction in the intraocular pressure from the baseline, at 6 weeks follow
up, measured on tonometry. All the relevant data was recorded in a pre-designed
printed proforma. Those patients who developed drug
side effects and those who don’t come for follow up were omitted from the
study. Confounders and bias in the study was controlled by strictly following
the inclusion and exclusion criteria. SPSS 10 was used for analysis of data. Efficacy
in terms of reduction of IOP was compared between travoprost
and FCDT. Mean ± standard deviation was calculated for quantitative variables;
percentage and proportion were calculated for qualitative variables. Chi-square
test was used to compare the efficacy in both groups. All the results were
presented as tables and charts in a meaningful way. (P-Value had generated
using student t-test for comparison of mean and chi-square test for comparison
of percentages. P-Value < 0.05 had considered significant.)
RESULTS
A total of 136 (68 in each group) patients
were included in the study. In Group A Mean age was 53
years with standard deviation ± 13.26 whereas in Group B mean age was 55 years
with standard deviation ± 14.31.
Gender distribution among two groups was analyzed as in Group A 38
(56%) patients were male and 30 (44%) patients were female where as in Group B
40 (59%) patients were male and 28 (41%) patients were female (Table 1).
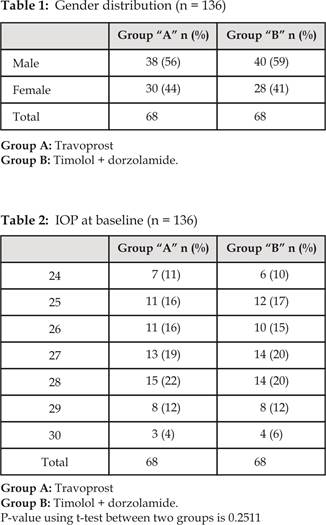
Baseline IOP (mm Hg) among two groups was
analyzed (Table 2). In Group A mean IOP was 27.05 mm Hg
with SD ± 1.8401. Where as in Group B mean IOP was 26.67 mmHg
with SD ± 2.0008 (Table 2).
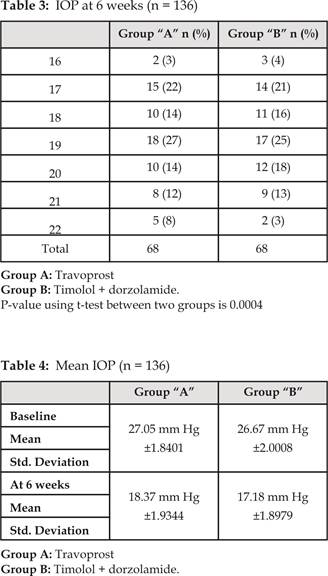
Status of IOP after 6 weeks among two groups was analyzed (Table
3). In Group A mean IOP was 18.37 mmHg with SD
±1.9344. Where as in Group B mean IOP was 17.18 mmHg with SD ±1.8979 (Table 3).
Comparison of mean baseline IOP and mean IOP at 6 weeks is shown in (Table 4).
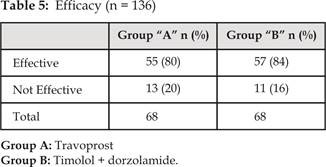
Efficacy of the two drugs was analyzed as travoprost
(Group A) was effective in 55 (80%) patients and was not effective in 13 (20%)
patients. Whereas Timolol+ Dorzolamide
(Group B) was effective in 57 (84%) patients and was not effective in 11 (16%)
patients (Table 5) (comparison of mean IOP is shown in (Table 4).
DISCUSSION
Open angle glaucoma can cause permanent
loss of vision. It remains asymptomatic and progress slowly until it is very severe
and irreversible damage has occurred in one or both eyes. It is the second most
common cause of irreversible blindness throughout the world2. A
number of risk factors are responsible for progression of glaucoma but
intraocular pressure IOP is currently the most important modifiable risk factor
that can be used to prevent progression of glaucoma. According to Early - Manifest
Glaucoma Treatment Study IOP reduction by at least 25% reduced progression
damage in the treated group from 62% to 45% compared to an untreated group13.
Mean intraocular pressure should be decreased to a patient dependent target
pressure in order to prevent progressive glaucomatous damage and to preserve
vision8. This target pressure depends on a number of
factors, including baseline IOP, age of patient, status of optic disc and nerve
fiber layer and functional damage assessed on visual field assessment11.
The main objective of management of glaucoma is to preserve the visual
functions and hence improve the individual’s quality of life. The main
treatment modality particularly of open angle glaucoma is medical treatment. Number
of drugs is available which lowers the IOP either by enhancing the aqueous
outflow or decreasing aqueous secretion. The main objective of medical
treatment is to maintain IOP at lower level according to patient’s target
pressure with the minimum possible concentration, fewer numbers of drugs as
well as using the safest drugs with limited local and systemic side effects11.
Most commonly used drugs to decrease intraocular pressure in glaucoma are
topical beta blockers. They are useful in all types of glaucoma and act by
decreasing aqueous secretion. This IOP lowering effect however, decreases with
time in approximately 10% of cases. This IOP lowering effect may be lost within
a few days (short time escape) or may take months (long term drift)5. Beta blockers can cause local as
well as systemic side effects including respiratory, cardiovascular, and
metabolic side effects5. Our study results are similar to the
results of some international studies, in one of two small (n 50 and 56),
single – blind, parallel-group, single - center studies, Parmaksiz
et al13 had showed that the IOP-lowering effect of dorzolamide 2%/timolol 0.5% used twice daily was greater
than that of travoprost 0.004% used once daily. The
reduction in mean diurnal IOP (average of measurements noted at 08:00, 10:00
and 16:00 hours) from baseline with dorzolamide 2%/timolol 0.5% was superior to that with travoprost
0.004% (11.5 vs. 9.3 mm Hg; P0.05) after 6 months of treatment. In another
single dose blind, parallel- group, single-center comparison Dorzolamide 2%/timolol 0.5% was
less effective than travoprost 0.004%. In the
parallel-group comparison, the reductions in mean diurnal IOP (average of
measurements made at 08:00, 12:00, 16:00 and 20:00 hours) from baseline were
significantly less with dorzolamide 2%/timolol 0.5%
than with travoprost 0.004% after both 3 weeks of
treatment (23.1% vs. 32.7%; P 0.01) and 6 weeks of treatment (21.7% vs.
30.7%; P 0.01). In a cross-over comparison, Franklin et al14
had shown that the decrease in mean diurnal IOP (average of measurements made
at 8:00 am, 10:00 am and 4:00 pm) from baseline following 3 months of treatment
with dorzolamide 2%/timolol 0.5% (14.3%; P 0.0001
vs. baseline) was significantly less than that with travoprost
0.004% (18.4%; P 0.0001 vs baseline) and dorzolamide 2%/timolol 0.5%) and latanoprost
0.005% (22.1%; P 0.0001 vs. baseline) and dorzolamide
2%/timolol 0.5%). The tolerability of a drug is the
main barrier to compliance as shown by Strohmaier K et al15. Local burning, stinging, discomfort, and
taste perversion are the most common adverse effects associated with
dorzolamide16. Kalzuny et al17 showed
in their study that dorzolamide 2%/ timolol 0.5% fixed combination twice daily was generally
well tolerated in large in large group of patients (n 177 – 492) given either
as monotherapy or concomitantly, trials of 3 to 6
months duration which evaluated this fixed combination in relation to the
individual components, or against other ocular hypotensive agents. In these
studies 33% and 77% of patients receiving dorzolamide
2%/timolol 0.5% reported adverse effects. 10% to 68% reported drug-related
adverse events. Most commonly reported ocular adverse event in majority of the
trials was transient mild to moderate burning and/or stinging of the eye (5% – 41%).
While the most common systemic adverse effect was dysgeusia
(2% – 38%)18. Teus
et al19 in their study compared timolol 0.5% and brinzolamide 1%.
The most common side effects with brinzolamide 1%
were blurred vision and taste perversion; while ocular discomfort was less
common. Manni et al20 in
their study showed that of the 106 subjects, 79.2% preferred brinzolamide 1%/timolol 0.5% (P 0.0001). Ocular
discomfort was significantly higher with dorzolamide
2%/timolol 0.5% than brinzolamide 1%/timolol 0.5%
(2.9 vs 1.4, respectively; P0.0001). With dorzolamide 2%/timolol 0.5%
instillation most common side effect was ocular pain and discomfort while with brinzolamide 1%/timolol 0.5% instillation it was transient
blurred vision. Manni et al20 observed in
his study that brinzolamide 1%/timolol 0.5% showed
significantly less ocular irritation (2.7% vs. 10.6%; P 0.0009) than dorzolamide 2%/timolol 0.5%.A statistically significant
difference in conjunctival hyperemia in travoprost 0.004%/ timolol 0.5%
group compared to dorzolamide 2%/ timolol
0.5% was shown by Teus et al in his study19.
In a non-blind extension of one study, fixed combination was generally well
tolerated for up to 1 year21. In 3 small, single-center studies, the
IOP-lowering effects of dorzolamide–timolol fixed combination therapy were shown to be both
better and worse than the efficacy of travoprost
0.004% monotherapy22-24. Fixed combination therapy with dorzolamide–timolol dosed twice
daily was less efficacious than monotherapy with travoprost 0.004% dosed once daily in patients with OAG or
OH as shown by Suzuki
et al22. IOP reduction and percentage of
IOP reduction were compared. Mean average IOP reductions from baseline at 3 and
6 weeks, were −7.5 mm Hg and −7.1 mm Hg respectively, for the travoprost monotherapy group and
−4.8 mmHg and −4.5 mm Hg at 3 and 6 weeks, respectively, for the dorzolamide–timolol fixed
combination therapy group. The better mean diurnal IOP reduction in the
patients receiving travoprost 0.004% monotherapy compared with those receiving dorzolamide–timolol fixed
combination therapy was statistically significant at both follow-up time points
(P < 0.01).
CONCLUSION
Our study concludes that dorzolamide 2% with timolol 0.5% combination
used twice daily is more efficacious than Travoprost
0.004% used once daily in primary open angle glaucoma.
Our results show that Timolol + Dorzolamide
cause reduction in IOP of at least 20% in 84% patients while Travoprost cause reduction in IOP of at least 20% in 80%
patients. Also p-value calculated at six week is statistically significant.
Author’s Affiliation
Dr. Farooq Khan
Trainee Medical Officer
Ophthalmology Department
Khyber Teaching Hospital, Peshawar
Dr. Mubashir Rehman
Medical Officer
Ophthalmology Department
Lady Reading Hospital, Peshawar
Dr. Omar Ilyas
Trainee Medical Officer
Ophthalmology Department
Khyber Teaching Hospital, Peshawar
Dr. Mohammad Zeeshan Tahir
Medical Officer
Ophthalmology Department
Lady Reading Hospital, Peshawar
Dr. Imran Ahmad
Vitreoretina Trainee
Ophthalmology Department
Hayatabad Medical Complex, Peshawar
Role of Authors
Dr. Farooq Khan
Patients’ selection, data collection and data analysis
Dr. Mubashir Rehman
Patients’ selection, data collection and data analysis.
Dr. Omar Ilyas
Patients’ selection, data collection and data analysis.
Dr. Mohammad Zeeshan Tahir
Literature search and references.
Dr. Imran Ahmad
Literature search and
references.
REFERENCES
1.
Kumarasamy NA,
Lam FS, Wang AL, Theoharides TC. Glaucoma: Current and developing concepts for inflammation,
pathogenesis and treatment. Eur J Inflamm.
2006; 4: 129-37.
2.
Quiiqley HA,
Broman AT. The number of people
with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262-7.
3.
Dineen B,
Bourne RR, Jadoon Z. Causes of blindness and visual impairment in Pakistan. Br J
Ophthalmol. 2007; 91: 1005-10.
4.
Hazin R, Hendrick AM, Kahook MY. Primary open-angle glaucoma: Diagnostic approach and management.
J Nati Med Assoc. 2009; 101: 46-50.
5.
Kanski JJ. Glaucoma. In: Kanski JJ Clinical
Ophthalmology. A systemic approach 6th ed. Butterworth Heinemann
Elsevier 2007; 371-440.
6.
Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat
Res. 2006; 612: 105-14.
7.
Theelen T, Meulendij CF, Geurts DE. Impact factors on intraocular pressure measurement in healthy
subjects. Br J Ophthalmol. 2004; 88: 1510-1.
8.
Lodhi AA, Talpur KI, Khanzada MA. Latanoprost 0.005% v/s timolol maleate 0.5% pressure lowering effect in primary
open angle glaucoma. Pak J Ophthalmol. 2008; 24 (2): 68-72.
9.
Macleod SM, Clark R, Forrest J, Bain M, Bateman N, Azuara-Blanco A. A review of glaucoma treatment in Scotland 1994-2004. Eye 2004; 22:
251-5.
10.
The European
Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma
Prevention Study. Ophthalmology. 2005; 112: 366-75.
11.
Parikh RS, Parikh SR, Navin S, Arun E, Thomas R. Practical approach to medical management of glaucoma. Indian J
Ophthalmol. 2008; 56: 223-30.
12.
Sharma R, Kohli K, Kapoor
B, Mengi RK, Sadotra P, Verma U. Comparative
effect of timolol, levobunolol
and betaxolol on IOP in patients of chronic simple
glaucoma. JK science. 2005; 7: 61-4.
13.
Parmaksiz S, Yuksel N, Karabas VL. A comparison of travoprost, latanoprost, and the fixed combination of dorzolamide and timolol in
patients with pseudoexfoliation glaucoma. Eur J Ophthalmol. 2006; 16: 73–80.
14.
Franklin LM, da Silva LJ. Comparison of the efficacy and safety of travoprost
with a fixed – combination of dorzolamide and timolol in patients with open – angle glaucoma or ocular
hypertension. Curr Med Res Opin.
2006; 22: 1799–805.
15.
Strohmaier K,
Snyder E, DuBiner H. The efficacy and safety of the dorzolamide-timolol
combination versus the concomitant administration of its components. Dorzolamide-Timolol Study Group. Ophthalmology. 1998; 105: 1936–44.
16.
Sevda AK, Semih A, Ahmet A, Nurver O, Tomris S, Osman O. The Effects of Topical Antiglaucoma
Drugs as Monotherapy on the Ocular Surface: A
Prospective Study. J. Ophthal. 2014: 112-120.
17.
Kalzuny J, Szaflik J, Czechowicz-Janicka K. Timolol 0.5%/ dorzolamide
2% fixed combination versus timolol 0.5%/pilocarpine
2% fixed combination in primary open angle glaucoma or ocular hypertensive
patients. Acta Ophthalmol Scand. 2003; 81: 349–54.
18.
Cvenkel B,
Stewart JA, Nelson LA, Stewart WC. Dorzolamide/timolol fixed combination versus latanoprost/timolol fixed combination in patients with primary
open-angle glaucoma or ocular hypertension. Curr Eye
Res. 2008; 33: 163–8.
19.
Teus MA, Miglior S, Laganovska G. Efficacy and safety of travoprost/timololvsdorzolamide/timolol in
patients with open-angle glaucoma or ocular hypertension. Clin
Ophthalmol. 2009; 3: 629–36.
20.
Manni G,
Denis P, Chew P. The safety and efficacy
of brinzolamide 1%/timolol 0.5% fixed combination
versus dorzolamide 2%/timolol 0.5% in patients with
open-angle glaucoma or ocular hypertension. J Glaucoma. 2009; 18: 293–300.
21.
Mundorf TK, Rauchman SH, Williams RD, Notivol
R. Brinzolamide/ Timolol Preference Study Group. A
patient preference comparison of Azarga (brinzolamide/timolol fixed
combination) vsCosopt (dorzolamide/timolol fixed combination) in patients with open-angle
glaucoma or ocular hypertension. Clin Ophthalmol.
2008; 2: 623–8.
22.
Suzuki ER.
Comparison of the efficacy and safety of travoprost
with a fixed-combination of dorzolamide and timolol in patients with open-angle glaucoma or ocular
hypertension. Curr Med Res Opin.
2006; 22: 1799-805.
23.
Nicholas PB, José LR, Robert MF. Safety, tolerability, and efficacy of fixed combination therapy
with dorzolamide hydrochloride 2% and timolol maleate 0.5% in glaucoma and ocular hypertension. Clin Ophthalmol. 2010; 4: 1331–46.
24.
Jin-WC, Shi-WC, Lian-DG, Guo-CL, Rui-LW. Pressure-Lowering Effects of Commonly Used Fixed-Combination
Drugs with Timolol: A Systematic Review and
Meta-Analysis. PLoS One. 2012; 7: e45079.